Our Approach to Alzheimer's Research
Alzheimer’s Disease Research, a BrightFocus Foundation program, takes a 360-degree approach to fund innovative scientific research worldwide, exploring the full range of scientific paths toward causes, treatments, and, ultimately, a cure for Alzheimer’s disease. By investing in a wide range of innovative scientific approaches—from biology and genetics to understanding risk reduction and new treatments—we leave no stone unturned in the quest for a cure.
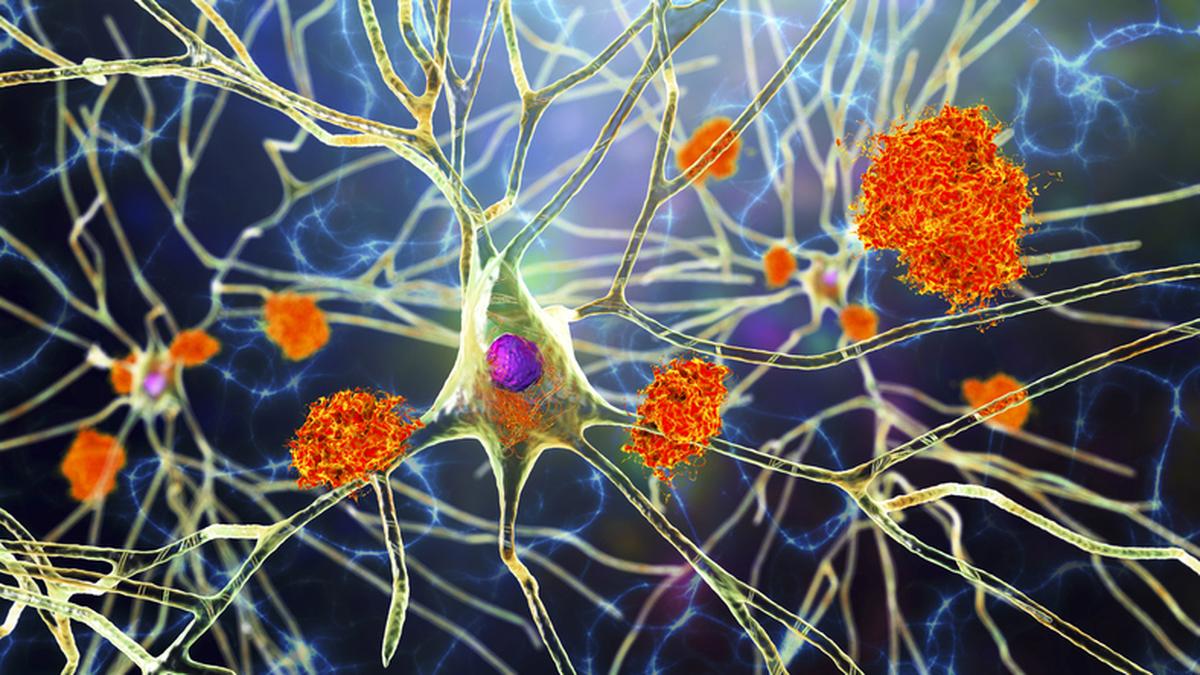
Amyloid-Beta
There are many versions of amyloid protein in the human body, and most serve a useful role. One type, amyloid-beta, or Aβ, is prone to fragmenting and accumulating in the brain. A healthy brain can break down the protein and eliminate it, but in Alzheimer’s disease, Aβ forms hard plaques that are toxic to neurons and sometimes (not always) associated with memory loss and other changes.
Many experts think Aβ may work synergistically with tau—another protein overexpressed in Alzheimer’s—to speed neurodegeneration.
With new technologies, researchers can directly measure amyloid plaques—the places where amyloid has accumulated in the brain—and identify affected brain regions, whereas previously, plaques could be seen only at autopsy.
Biology of Fats & Proteins (APOE)
Initially recognized for its role in cardiovascular disease, the apolipoprotein E, or APOE, gene (indicated by italics) also is a risk factor in Alzheimer’s disease. It produces the APOE protein (indicated by no italics), which metabolizes and transports fats (lipids) in the body.
Among the common variants of APOE, APOE4 (ε4) is the most prevalent genetic factor associated with late-onset Alzheimer’s. Its impact varies based on whether the mutation appears on one or both chromosomes and by race and ethnicity, which scientists are trying to better understand.
Some clues may lie with the protein’s ability to transport fats and interactions within the immune system, where it influences inflammation and a type of cellular damage known as oxidation. It helps facilitate the breakdown of Aβ protein in and around neurons, but the ε4 version of the protein is less effective at doing so.
Biomarkers
Biomarkers are biological indicators that are detectable through minimally invasive procedures to measure changes associated with Alzheimer’s disease. Because Alzheimer’s-related brain changes can arise 10-20 years before symptoms appear, researchers are focusing on novel biomarkers that support detection in earlier disease stages. The best hope of stopping Alzheimer’s is to identify it as early as possible.
Alzheimer’s biomarkers include elevated blood or cerebrospinal fluid amyloid-beta levels, changes in brain structure and function on advanced neuroimaging, and vascular changes or protein deposits in the retina that mirror those in the brain.
Biomarkers can be used to identify people likely to develop Alzheimer’s, track disease progression and treatment efficacy, and identify who needs treatment, when to start it, and what drugs and other strategies will be most successful.
Cells & Circuits
The human brain has an estimated 100 billion neurons. Extending from each is a long fiber, or axon, that can stretch several feet. Each axon forms a connection, or synapse, with another neuron, creating a circuit for brain signaling.
In Alzheimer’s disease, neurons die and do not regenerate, but some brains will remodel to meet new demands from these losses. If a circuit is too damaged to connect by a direct route, signaling can take detours or indirect neural pathways.
Classic Alzheimer’s disease symptoms—forgetting loved ones or becoming lost in familiar places—will emerge only when the communications network breaks down completely.
Scientists are studying the brain’s many cells and circuits, looking for ways to preserve communications for as long as possible after Alzheimer’s disease onset.
Genomics: DNA Blueprint for Alzheimer’s
Genes carry the instructions that cells use to make proteins, which build, operate, and repair tissue. Even slight changes to these instructions can result in an abnormally functioning protein, possibly affecting an individual’s risk of a disease like Alzheimer’s.
Only early-onset Alzheimer’s disease, however, is consistently linked to mutations in known genes, representing about 10% of cases. The remaining 90% are associated with genetic risk variants scattered throughout the genome.
Researchers use powerful technologies to find genetic variations, patterns, and interactions in people with and without Alzheimer’s. Other factors, such as environment and lifestyle, also can affect risk.
Genomics studies focus on triggers of Alzheimer’s disease, how genes interact with the environment to influence risk, who is most at risk and might benefit from new treatments, and how treatments can be individually tailored.
Immunity & Inflammation
One theory about Alzheimer’s disease is that a breakdown in the brain’s immune system may play a role. Normally, cells can “take out the garbage”—clear damaged cells and unwanted particles and dispose of them into the bloodstream.
However, a chronic buildup of debris, including toxic amyloid-beta and tau proteins, can short-circuit the cleanup process, causing chronic inflammation and cell damage.
Microglia are the brain’s primary immune cells. Under normal conditions, they maintain equilibrium, clear unwanted particles, and respond to injuries and infections. In Alzheimer’s, they acquire a disease-associated profile and release proinflammatory molecules that exacerbate disease mechanisms, including protein misfolding and energy dysfunction.
Researchers are looking at the causes of this imbalance and how to support cells, including microglia, in fighting Alzheimer’s.
Metabolism & Bioenergetics
The brain consumes 20% of the body’s total energy supply, more than any other organ. With no reserve energy supplies to draw on, it depends on reliable metabolic function to convert glucose and/or alternative fuel sources, such as glycogen, ketone bodies, and amino acids, into usable power.
Unfortunately, glucose metabolism declines with aging, and this decline is one of the earliest and most consistent events in Alzheimer’s disease. Metabolic dysregulation impacts brain processing speeds and cognition, and dysfunctional mitochondria (known as the “power plants” of cells) contribute to a neurotoxic environment by releasing reactive oxygen species and other molecules that evoke inflammatory responses in microglia.
Other Misfolded Proteins
Most dementia diagnosed in people over 80 years old is of mixed etiology, meaning that it results from more than one disease or cause. Misfolded proteins and other disease mechanisms interact to exacerbate disease pathology and worsen clinical symptoms, including cognitive impairment.
Many misfolded proteins are hallmarks of other diseases, such as frontotemporal dementia, amyotrophic lateral sclerosis (ALS), and Parkinson’s disease, and the presence of a second misfolded protein in Alzheimer’s, in addition to amyloid, increases the extent of cognitive impairment.
Vascular dementia, in which blood circulation to the brain is compromised, is another frequent contributor to mixed dementia. Clinically distinguishing between dementias is important for therapies that target specific proteins.
Research Tools & Resources
When embarking on a research project, having the right preliminary information and tools can make or break its success, especially in understudied areas, and taking the first steps requires time and funding. Alzheimer’s Disease Research grants support the development of resources used to conduct, translate, and disseminate high-quality dementia research, including shared data and tissue repositories, along with collaborative projects aimed at accelerating new knowledge, disease models, and interventions.
Sex-Based Differences
Women are disproportionately affected by Alzheimer’s and account for two-thirds of Alzheimer’s-related diagnoses. At one time, their greater disease burden was attributed to their longer life expectancies compared with men, given that age is the greatest risk factor for the disease. But in recent years, additional sex-based differences in genetics and immunity have been identified. Hormones and underlying risks—such as higher rates of depression in women than men—are actively being explored at all levels of Alzheimer’s research, from cell studies to epidemiology to treatment trials.
Sleep & Circadian Rhythm
Chronic sleep disruption plays a role in Alzheimer’s disease. The brain depends on nightly deep sleep to flush waste material and toxins, including misfolded proteins, and break down and dispose of amyloid-beta plaques present in early Alzheimer’s. Sleep patterns are governed by circadian rhythms, the body’s 24-hour master clock that synchronizes essential functions, including the sleep-wake cycle. This biological clock is influenced by environmental cues, including light, and possibly by factors tied to neurodegeneration. Changes in sleep patterns are common in Alzheimer’s, even before cognitive disturbances. A major research question is whether sleep disruption is a symptom of Alzheimer’s-related neurodegeneration and can serve as an early warning, or whether it’s a contributing factor that can be treated.
The Impact of Tau
Tau is another protein associated with Alzheimer’s disease. Found abundantly inside neurons, its fibrous shape lends stability to tubes that transport nutrition and waste in and out of the brain. However, in Alzheimer’s, tau goes through molecular changes that cause it to become misshapen and collect in messy tangles. Unlike amyloid plaques, which can form years and even decades before Alzheimer’s symptoms occur, tau tangles typically are a sign that Alzheimer’s is rapidly getting worse. Current theories hold that misfolded amyloid-beta and tau proteins interact in ways that lead to disease progression, and scientists are investigating how tau is involved in spreading Alzheimer’s throughout the brain.
Translational Research & Clinical Interventions
Translational research refers to the effort to take basic science knowledge from the lab or research setting into the real world as potential treatments or cures—to “translate” science into useful ways of diagnosing, treating, and managing Alzheimer’s disease.
This translation can take many different forms, from using smartphone-based testing to monitor cognitive status to finding ways to improve sleep and exercise to promoting lifestyle activities associated with brain health as potential protective benefits. The essential process of testing new drugs and interventions relies on volunteers who participate in clinical trials and other studies. These activities help speed drugs, treatments, and critical knowledge to the marketplace and put them in the hands of people living with Alzheimer’s today and those at risk in the future.
Vascular Contributions to Dementia
Vascular dementia is the most common condition co-occurring with Alzheimer’s disease. To survive and function properly, neurons depend on oxygen and glucose carried through the brain’s blood vessels, or vascular system. Their needs are great because the brain consumes more energy than any other organ. The brain relies heavily on an intricately laced system of arteries, veins, and capillaries that, in adults, stretches out an estimated 60,000 miles.
For protection, the brain’s circulatory system is sealed off from the rest of the body by a blood-brain barrier that helps prevent bacteria, viruses, and other toxic substances from entering. Together, the brain’s circulatory system and protective barrier are important to Alzheimer’s research and are key to keeping neurons healthy.
Waste-Clearance Mechanisms
Along with the immune-mediated clearance duties of the microglia, individual cells undergo a process of self-eating, or autophagy. By cleaning out old, used, or misprocessed proteins, a cell can self-rejuvenate to optimize health and function.
Endosomes and lysosomes are cellular compartments responsible for breaking down and recycling or removing waste. Endo-lysosomal pathway abnormalities are an early Alzheimer’s disease feature and are closely related to genetic risk factors.
The brain has its own lymphatic system associated with its membranes, or meninges. The meningeal lymphatics drain fluid from within and around the brain and carry immune cells to and from the brain. Impaired clearance of toxic proteins through meningeal lymphatics is not only a likely contributor to Alzheimer’s pathology but also a novel target for therapeutic interventions.
News
Explore Our Research

Article
Can Targeting the Immune System Slow the Progression of Alzheimer's?
BrightFocus Alzheimer’s Disease Research grant recipient Joshua Emmerson, PhD, is exploring a certain type of immune cell’s unexpected role in Alzheimer’s disease—and what it could mean for protecting the brain.

Article
Unlocking the Brain's Regenerative Potential to Fight Alzheimer's
María Llorens-Martín is investigating why the brain stops making new cells in Alzheimer’s—and how fixing that process could change lives.
Article
Breaking News Dispatch: New Findings on GLP-1 Drugs and Alzheimer's, Novel Treatments in Development, and More
Get the latest news from the 2025 Clinical Trials on Alzheimer’s Disease (CTAD) conference.

Article
4 Takeaways from Alzheimer's Fast Track 2025
This innovative meeting hosted by BrightFocus Foundation bridges cutting-edge science, mentorship, and community building for early-career researchers.

Article
Understanding Alzheimer's & Exploring New Frontiers in Treatment
Watch a conversation inspired by the documentary film Taking Care and the latest research in Alzheimer’s therapies.

Article
Shaping the Future of National Alzheimer’s Research Priorities
BrightFocus Foundation’s VP of Scientific Affairs helped set national research priorities at the federal Alzheimer’s Disease-Related Dementias Summit, influencing how billions in NIH funding will advance dementia research and care.

Article
New Clues in the Fight Against Alzheimer's: Q&A with Dr. Keith Hengen
During World Alzheimer’s Month, Alzheimer’s Disease Research grantee Dr. Keith Hengen joined BrightFocus’ Dr. Sharyn Rossi on Instagram Live to talk brain health—how the brain functions, why quality sleep matters, and tips to keep your brain healthy.

Article
Targeting Genetic Triggers of Inflammation in Alzheimer's Disease
A BrightFocus Alzheimer’s Disease Research-funded scientist is investigating how “jumping genes” may fuel brain inflammation in Alzheimer’s—and whether blocking them could prevent or slow dementia.

Article
FDA Approves At-Home Injectable Leqembi for Alzheimer's
New formulation of Leqembi allows weekly at-home injections, reducing the need for hospital visits.


