Tau Protein and Alzheimer's Disease: What's the Connection?
Written By: Caleigh Findley, PhD
Reviewed By: Sharyn Rossi, PhD, Senior Director, Neuroscience Programs, BrightFocus Foundation
Written By: Caleigh Findley, PhD
Reviewed By: Sharyn Rossi, PhD, Senior Director, Neuroscience Programs, BrightFocus Foundation
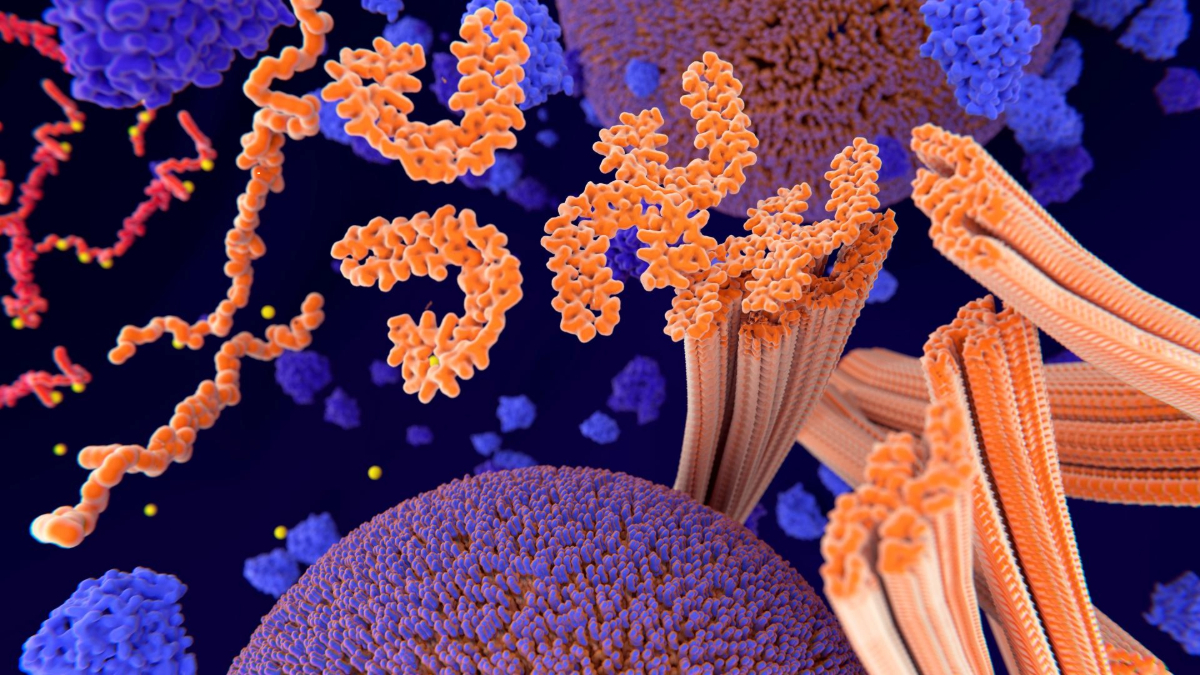
Tau is a small protein with a short name and a big reputation. Predominantly found in the brain, this protein serves multiple functions in healthy brain cells.
Like most proteins, tau is vulnerable to changes in its environment and genetic mistakes. These factors can make tau no longer fit to carry out its usual job—a problem associated with many brain diseases. A buildup of the abnormal tau leads to “tangles” that cause cell damage and inflammation, contributing to Alzheimer’s disease symptoms.
Read on to unpack the role of tau in Alzheimer’s disease.
Tau is found mostly in the messenger cells of our brains, called neurons. We need these cells to communicate with one another for even the most basic of tasks in our daily life.
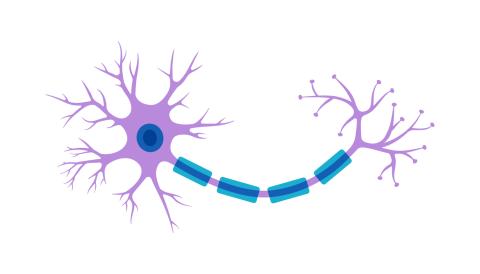
Neurons have a peculiar shape, that can be divided into three areas: a messaging-receiving end, a long hallway, and a message-sending end. Inside, we find a superhighway system that transports important molecules back and forth across the cell.
This shuttle system must span the long hallway, or axon, of the neuron. To do this, the cell has a “skeleton” of tube-like structures called microtubules that make up the superhighway. Tau stabilizes these microtubules to help them function best.
Smaller forms of tau, called oligomers, also exist in the spaces between neurons. In high levels, this can impact communication between brain cells and throw them off balance. Elevated tau levels are observed in the brain decades before the onset of Alzheimer’s disease symptoms like memory loss.
In Alzheimer’s disease, an abnormal form of tau accumulates and begins sticking together in thread-like structures called neurofibrillary tangles. These tangles are not effectively disposed of through the cell’s usual ways of removing “trash.” The build-up damages the microtubule superhighway, disrupting how neurons function.
Tau tangles start in the brainstem, which connects the brain to the spinal cord. From the brainstem, they spread upward toward two brain regions that are key to memory.
The first stop is the entorhinal cortex: a gateway for the learning center of the brain. Some of the earliest damage in Alzheimer’s disease is seen in the brain cells here. From there, it progresses into the learning center, called the hippocampus. This is where the brain figures out something new, like the rules of a board game, and creates a short-term memory.
The accumulation of tangles in these regions is one of several biological outcomes in Alzheimer’s disease that leads to memory loss and other symptoms. The amount of abnormal tau in the brain is linked to disease stage and severity. Tracking tau levels is a reliable predictor, and earlier indicator, of cognitive decline.
Learn more in the video below.
Video courtesy of the National Institute on Aging/National Institutes of Health
Thanks to advances in science, tau levels can provide clues into Alzheimer’s disease progression—a process that can span 20 years or longer. The earlier the disease can be identified, the more potential options for treatment available.
Two commercially available blood tests can detect abnormal tau in Alzheimer’s disease. These tests measure tau that has undergone molecular changes, known as hyperphosphorylation, creating abnormalities associated with Alzheimer’s disease.
Specialized imaging tools can also be used to detect tau. First, a physician will inject a brain tau-binding agent (“tracers”) into a vein so it can be visualized. To see the tau, doctors use a positron emission tomography (PET) scan, which picks up a signal from the tracer. Clinicians can then look at images from these scans to see the amounts and locations of tau in the brain.
PET scans for tau can provide a biomarker measurement that meaningfully indicates disease progression. In 2020, the U.S. Food and Drug Administration approved the first tau PET tracer (F-18 flortaucipir). Many Alzheimer’s clinical trials now include tau PET scans as a potential biomarker of disease severity.
This hallmark protein represents one of the many ways that Alzheimer’s disease can harm your brain cells and cause symptoms like memory loss. Researchers are harnessing this information for early detection and diagnosis to empower earlier treatment and better patient outcomes for the future.
BrightFocus Foundation is a premier global nonprofit funder of research to defeat Alzheimer’s, macular degeneration, and glaucoma. Since its inception more than 50 years ago, BrightFocus and its flagship research programs—Alzheimer’s Disease Research, Macular Degeneration Research, and National Glaucoma Research—has awarded more than $300 million in research grants to scientists around the world, catalyzing thousands of scientific breakthroughs, life-enhancing treatments, and diagnostic tools. We also share the latest research findings, expert information, and resources to empower the millions impacted by these devastating diseases. Learn more at brightfocus.org.
Disclaimer: The information provided here is a public service of BrightFocus Foundation and is not intended to constitute medical advice. Please consult your physician for personalized medical, dietary, and/or exercise advice. Any medications or supplements should only be taken under medical supervision. BrightFocus Foundation does not endorse any medical products or therapies.
Every Donation is a Step Forward in the Fight Against Alzheimer’s
Your donation powers cutting-edge research and helps scientists explore new treatments. Help bring us closer to a cure and provide valuable information to the public.
Donate Today