Researchers Identify Gene Pathway for Repairing and Regenerating Retinal Cell
Written By: BrightFocus Editorial Staff
Written By: BrightFocus Editorial Staff
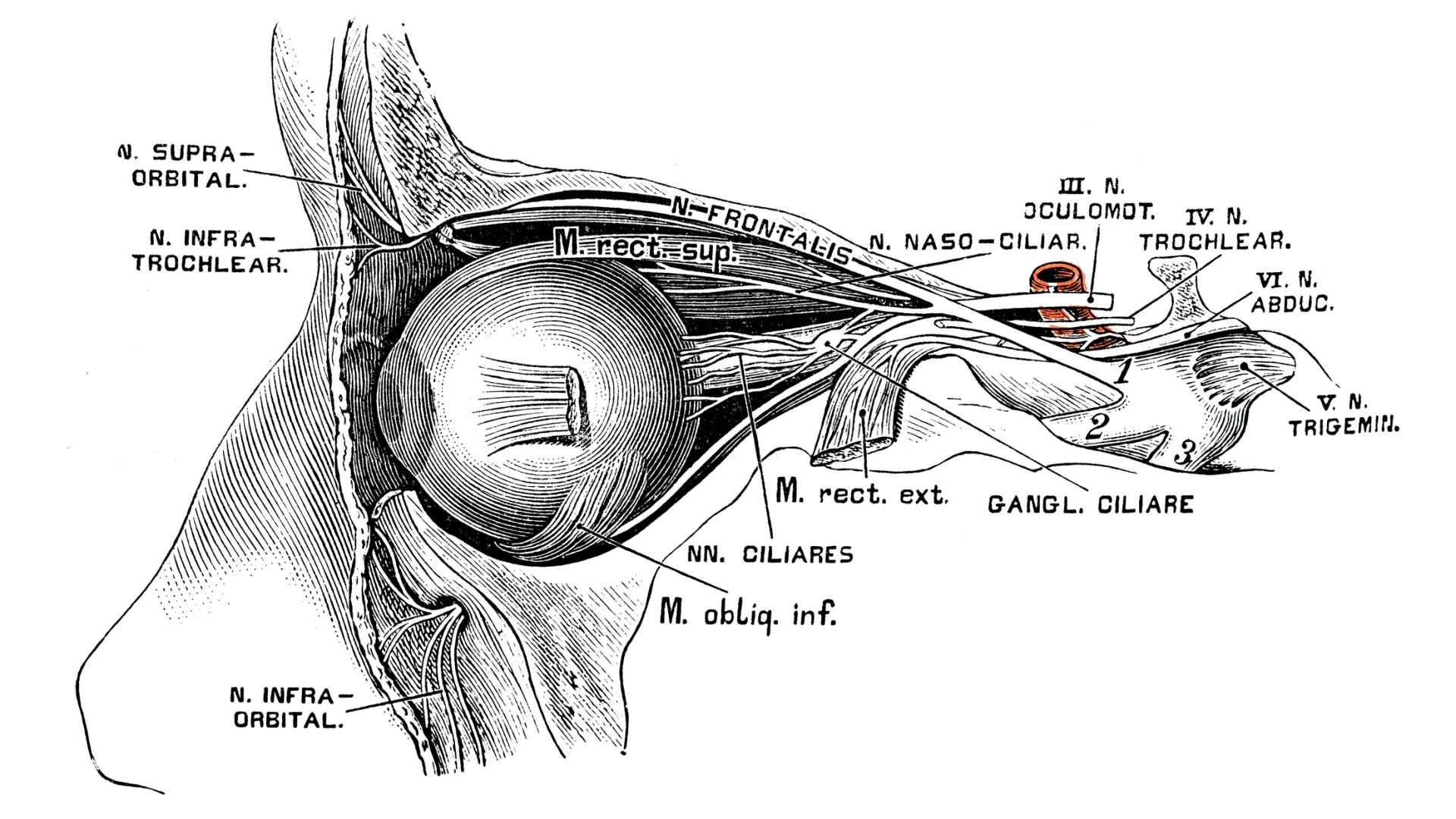
What: In humans and other mammals, retinal ganglion cells and other neurons no longer regenerate once development is complete (ie, soon after birth). Scientists identified a key gene that regulates this process and are hoping they can turn RGC regeneration back on to preserve vision in glaucoma.
Where: Lukomska A et al., Developmentally Upregulated Transcriptional Elongation Factor A Like 3 Suppresses Axon Regeneration After Optic Nerve Injury, Neuroscience Letters, 2021.
BrightFocus Connection: This work was funded in part by a BrightFocus National Glaucoma Research (NGR) grant to Ephraim F. Trakhtenberg, PhD, senior author, who is an an assistant professor of neuroscience at the University of Connecticut Medical School.
Why It Is Important: Glaucoma is characterized by damage and eventual death of retinal neurons that process visual information, which are called retinal ganglion cells (RGCs). Each RGC has a long tail (called an axon) extends from the retina into the brain. RGC axons exit the eye in a bundle through the optic nerve. One of the modifiable risk factors for vision loss from glaucoma is a chronic rise in fluid pressure inside the eye (aka, intraocular pressure, or IOP), which can damages the optic nerve, ie, successively first destroying the outermost axons in the bundle, which are associated with peripheral vision, and eventually reaching the inner layer to cause severe blindness. RGCs and other nerve cells in the adult retina typically do not regenerate following damage.
Most current glaucoma therapies are directed towards lowering IOP; however, strategies to directly protect and/or save axons may also help preserve vision in glaucoma. With this goal in mind, Dr. Trakhtenberg and colleagues are working to identify gene products within RGCs that regulate the process of regeneration, genes whose function shuts down not long after birth. The hope is that it may be possible to modulate this pathway in order to restore the regenerative capacity of the eye.
A gene whose product inhibits regeneration would be expected to be expressed at greater levels in cells that regenerate poorly, such as following aging or injury. To find such a gene, the researchers analyzed embryonic, neonatal, and adult (both injured and uninjured) RGC gene expression libraries. They focused on a family of regulatory genes that are expressed in neurons and that in previous studies have been associated with regeneration of axons following injury. One member of this gene family, called transcription elongation factor a like 3 (Tceal3) was found to be expressed at low levels in RGCs from mouse embryos but at progressively higher levels in neonatal and adult RGCs. Expression of Tceal3 was further enhanced following injury to the optic nerve.
To test the influence of Tceal3 on regeneration, Trachtenberg’s group treated mice with a compound RNA designed to inhibit Tceal3 levels in RGCs, and then induced an injury to the optic nerve. Mice treated with the inhibitory compound demonstrated a significant increase in RGC axon regeneration compared to control mice.
This study points to a potential new, innovative approach to rescuing injured nerve cells in the early stages of the disease, while the cells are still alive, by down-regulating genes that inhibit regeneration. The potential exists to use this strategy for treating spinal cord injury and stroke, in addition to glaucoma.
BrightFocus Foundation is a premier global nonprofit funder of research to defeat Alzheimer’s, macular degeneration, and glaucoma. Since its inception more than 50 years ago, BrightFocus and its flagship research programs—Alzheimer’s Disease Research, Macular Degeneration Research, and National Glaucoma Research—has awarded more than $300 million in research grants to scientists around the world, catalyzing thousands of scientific breakthroughs, life-enhancing treatments, and diagnostic tools. We also share the latest research findings, expert information, and resources to empower the millions impacted by these devastating diseases. Learn more at brightfocus.org.
Disclaimer: The information provided here is a public service of BrightFocus Foundation and is not intended to constitute medical advice. Please consult your physician for personalized medical, dietary, and/or exercise advice. Any medications or supplements should only be taken under medical supervision. BrightFocus Foundation does not endorse any medical products or therapies.

University of Connecticut Health Center
Support Groundbreaking Glaucoma Research
Your support helps fund critical research that could prevent vision loss, provide valuable information to the public, and cure this sight-stealing disease.
Donate Today