Explore key milestones and see how our researchers are improving lives.
1985
Alzheimer’s Disease Research, a program of BrightFocus Foundation, begins.
1992
Researchers uncover new genetic causes of inherited Alzheimer’s disease.
Scientists including Alzheimer’s Disease Research grantees Drs. Rudolph Tanzi, Gerard Schellenberg, John Hardy, and Peter St. George-Hyslop discover a new area of chromosome 14 associated with familial Alzheimer’s disease, setting the stage for an improved understanding of hereditary genes for Alzheimer’s.

Dr. Gerard Schellenberg (left) and colleagues at the University of Washington.
1993
Key gene identified as a risk factor for Alzheimer’s.
Alzheimer’s Disease Research-funded scientists Drs. Allen Roses, Peter St. George-Hyslop, Thomas Bird, and Gerard Schellenberg identify apolipoprotein E (APOE) as a major risk factor for late-onset Alzheimer’s disease. Today, APOE is the most common gene associated with Alzheimer’s.
1993-1995
Alzheimer’s research expands in Europe.
BrightFocus helped established Alzheimer’s research programs in the Netherlands (1993), Germany (1995), and Belgium (1995), broadening philanthropic support for Alzheimer’s research internationally.

BrightFocus-supported Alzheimer’s research in Europe begins in the Netherlands in 1993.
1997
Stanley Prusiner, MD, receives Nobel Prize for groundbreaking prion discovery with links to Alzheimer’s disease.
Grantee Stanley Prusiner, MD, was awarded the 1997 Nobel Prize in Physiology or Medicine for his groundbreaking discovery and definition of prions, infectious proteins in the brain that cause mad cow disease in cattle and Creutzfeldt-Jakob disease in humans. There are similarities between the loss of brain function in prion diseases and in Alzheimer’s disease, and an understanding of how prion diseases begin and develop may one day lead to a treatment and a cure for Alzheimer’s. Dr. Prusiner served as co-chair of our Alzheimer’s Disease Research Scientific Review Committee and became an honorary BrightFocus board member in 2007. In 2010, President Obama awarded him the National Medal of Science, the country’s highest honor for science and technology.

Dr. Stanley Prusiner
1997
Stanley Prusiner, MD, receives Nobel Prize for groundbreaking prion discovery with links to Alzheimer’s disease.
Dr. Prusiner Accepts the BrightFocus Award for Scientific Impact
2000
Paul Greengard, PhD, receives Nobel Prize for his foundational insight that nerve cells communicate with each other.
A 1986 Alzheimer’s Disease Research grant led Paul Greengard, PhD, to initiate a series of milestone experiments into protein phosphorylation in specific brain regions related to Alzheimer’s disease. His most famous discovery, for which he was awarded the Nobel Prize in Physics or Medicine in 2000, is that nerve cells communicate with each other through chemical and electrical signaling working in tandem, known as signal transduction. This discovery laid the foundations of modern neuroscience.

Dr. Paul Greengard
2003
Researchers develop first optical test to diagnose Alzheimer’s disease.
Following his exciting discovery that Alzheimer’s disease can be detected in the lens of the eye, Lee Goldstein, MD, PhD, and his team developed new optical tests that can potentially diagnose and monitor the disease from the beginning stages, a discovery that could lead to an accessible, noninvasive way to detect, track, and treat Alzheimer’s.

Dr. Lee Goldstein (far right) and his team.
2005
BrightFocus expands Alzheimer’s research in Europe through the establishment of Alzheimer’s France.
Alzheimer’s research in Europe expands to France.
2008
Launch of educational workshop for early-career scientists.
BrightFocus offers its first workshop dedicated to Alzheimer’s disease research, now known as Alzheimer’s Fast Track, for PhD students and postdoctoral fellows to learn from world-renowned experts in the field. Today, the program includes Macular Fast Track and Glaucoma Fast Track and has educated thousands of early-career scientists from around the world.

Alzheimer’s Fast Track students receive feedback on their mock grant proposal from Laura Cox, PhD, assistant professor in the department of neurology at the Ann Romney Center for Neurologic Diseases at Harvard Medical School and Brigham and Women’s Hospital, one of the program’s speakers.
2008
Launch of educational workshop for early-career scientists.
2013
Expanding research into sex-based differences in Alzheimer’s disease.
Three BrightFocus-funded investigators win an award to investigate gender-based differences in Alzheimer’s disease diagnosis and treatment. Their work examines whether certain genes are expressed differently in men and women to explain the increased risks for Alzheimer’s in women and explores expanding technology to better predict sex-based differences in Alzheimer’s disease.
BrightFocus researchers explore the role of gender in Alzheimer’s.
2015
Bringing rigorous science to caregiving.
BrightFocus convenes the first national research summit on dementia caregiving, recognizing the evidence behind home-based dementia care as a critical component of care services for people with Alzheimer’s disease and related dementias in the coming decades.
A collaborative grant awarded in 2020 to Quincy Samus, PhD, would later advance the dissemination and translation of MIND at Home, an evidence‐based dementia care coordination model, into practice.

Dr. Quincy Samus
2015
Closer to the possibility of a vaccine to help rid the brain of toxic tau.
A team led by Alzheimer’s Disease Research grantees Kristen Funk, PhD, and Marc Diamond, PhD, brings us closer to the possibility of a vaccine by researching antibody immunotherapies to help rid the brain of toxic tau—and possibly keep it from spreading—in Alzheimer’s disease. Results of in vitro experiments show that monoclonal antibodies they’ve developed can disarm the harmful effects of abnormal tau protein, laying the groundwork for present-day clinical trials using anti-tau antibodies.

Kristen Funk, PhD, and Marc Diamond, PhD
2017
First citizen science online game speeds up Alzheimer’s research.
To accelerate Alzheimer’s research, BrightFocus grantee Pietro Michelucci, PhD, created the first-ever citizen science online game Stall Catchers, where participants look at videos from the brains of mice and try to identify vessel blockages, or “stalls,” to help researchers at Cornell University. Scientists have pinpointed the link between stalls and Alzheimer’s disease in mouse models and have successfully reversed some symptoms by reducing the number of stalls. While documenting these stalls is very time-consuming for researchers, citizen scientists playing the BrightFocus-funded Stall Catchers have drastically increased research output.
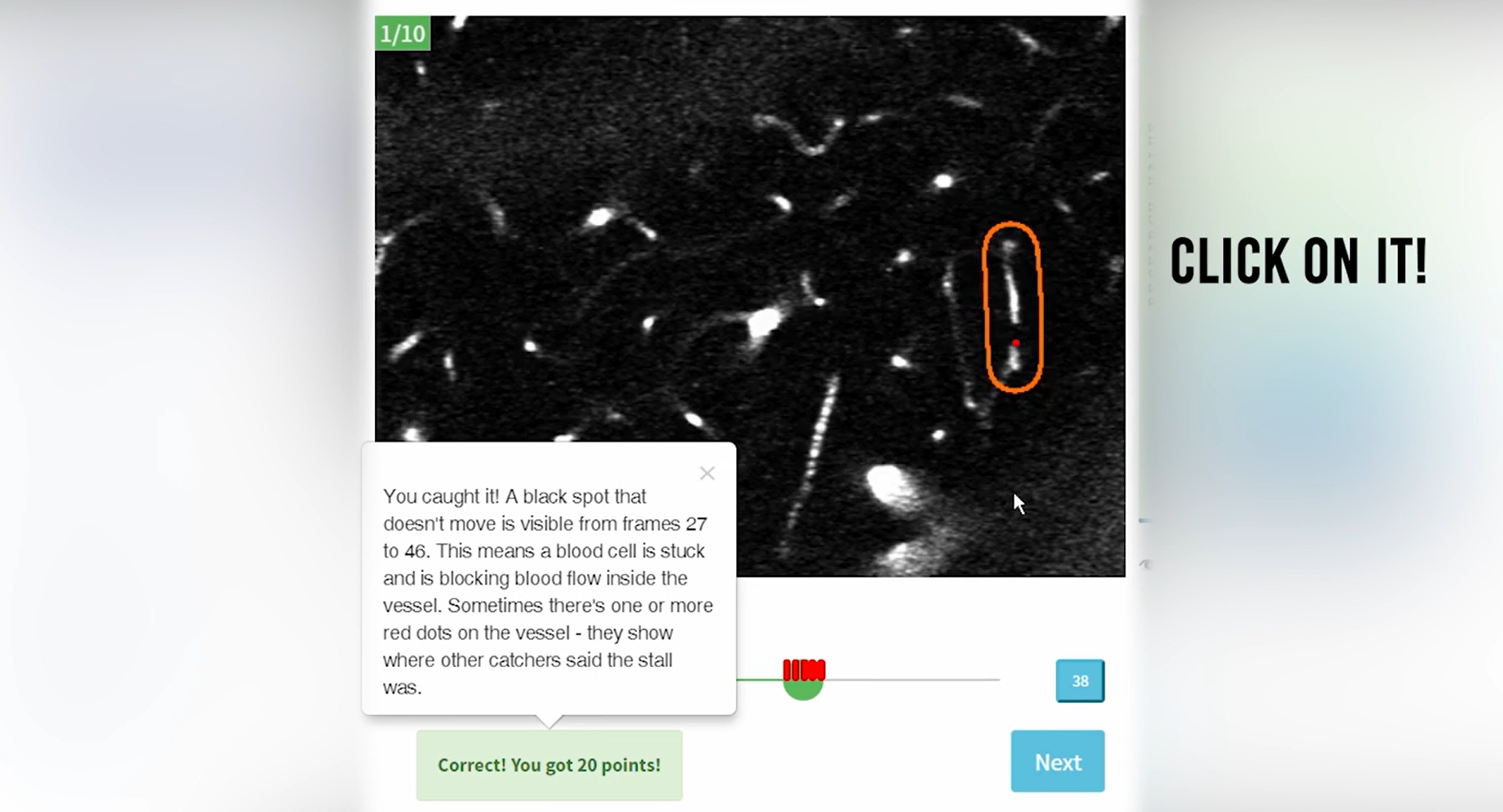
The online game Stall Catchers accelerates Alzheimer’s research.
2017
Scientists discover genetic causes and biochemical mechanisms of two rare inherited neurodegenerative diseases.
Grantee Huda Y. Zoghbi, MD, wins a Breakthrough Prize in Life Sciences for her discoveries of the genetic causes and biochemical mechanisms of two rare inherited neurodegenerative diseases, spinocerebellar ataxia and Rett syndrome. These findings help scientists better understand other neurodegenerative and neurological diseases, including Alzheimer’s, and led Dr. Zoghbi to pursue dementia research, for which she was later awarded a BrightFocus grant.

Dr. Huda Y. Zoghbi (left) and colleague.
2017
Creation of first-ever International Brain Bank for Down Syndrome-Related Alzheimer’s Disease.
With BrightFocus support, grantee Ann-Charlotte “Lotta” Granholm-Bentley, PhD, DDS, created the first-ever International Brain Bank for Down Syndrome-Related Alzheimer’s Disease, a collaborative research network that develops and shares high-quality biological research samples and studies information about the Down syndrome population, of which as many as 80% have Alzheimer’s pathology by the time they are in their 40s and 50s.

Dr. Lotta Granholm-Bentley
2019
New Alzheimer’s model reveals genetic changes associated with cell-aging processes.
Grantee Jerome Mertens, PhD, successfully reprograms cultured skin cells from Alzheimer’s patients directly into brain neurons, creating a brain model that is not only genetically unique to each patient but also biologically “remembers” the age of the individual. This research could lead to giving Alzheimer’s neurons their own memory back, paving the way for therapeutics to prevent or treat the disease.

Dr. Jerome Mertens (second from right) and colleagues.
2019
Sleep deprivation confirmed as a risk factor for Alzheimer’s.
In a seminal BrightFocus-funded publication on sleep and beta amyloid, coauthors and grantees Sarah Fritschi, PhD, Brendan Lucey, MD, and David Holtzman, MD, PhD, are among the first to confirm that sleep deprivation is a risk factor for—not just a consequence of—Alzheimer’s disease. The findings reveal that lack of sleep alone helps drive the disease, suggesting that good sleep habits may help preserve brain health.
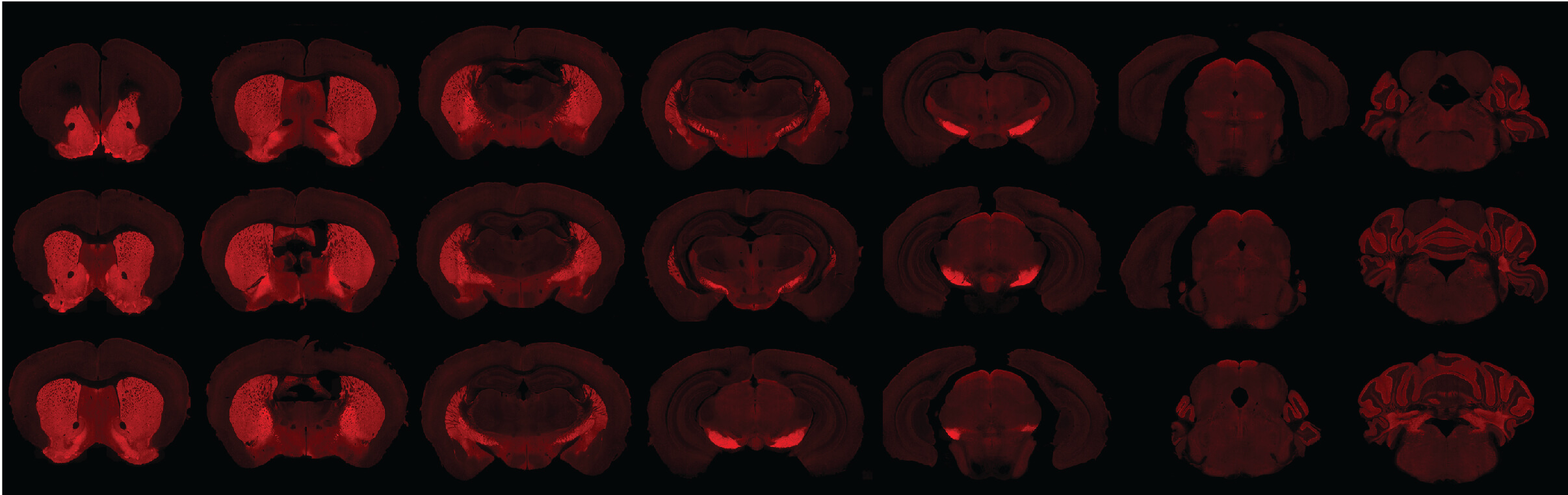
GABAergic neurons that play a critical role in sleep are pictured in red in a mouse brain. Photo courtesy of Sarah Fritschi, PhD.
2020
First Alzheimer’s blood test becomes available in the U.S.
C2N Diagnostics introduces the first widely accessible blood test in the U.S. to identify early signs of Alzheimer’s disease. After receiving ritical early support from BrightFocus, the PrecivityAD blood test received an FDA “Breakthrough Device” designation, paving the way for new treatments for earlier and better patient care.

The PrecivityAD blood test identifies early signs of Alzheimer’s. Photo courtesy of C2N Diagnostics.
2020
Deep sleep protects against Alzheimer’s, new research shows.
A team including grantee Ksenia Kastanenka, PhD, finds that sleep quality may serve as a marker of future Alzheimer’s disease risk and the speed of progression. Sleep disturbance is associated with a higher rate of future beta-amyloid accumulation and deep sleep can clear toxic beta-amyloid and tau from the brain, the researchers confirm.
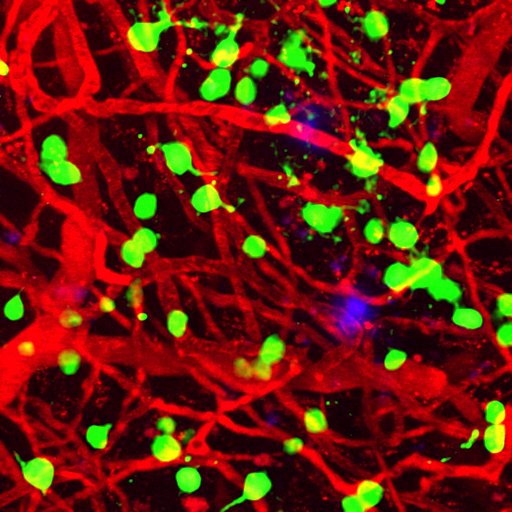
Amyloid plaques (blue) appear among brain blood vessels (red) and cortical neurons (green). Deep sleep can clear Alzheimer’s-causing amyloid plaques from the brain. Photo courtesy of Ksenia Kastanenka, PhD.
2020
Blood vessel changes in the eye may reveal early dementia.
By screening Alzheimer’s through the eye, a research team led by grantee Amir Kashani, MD, PhD, finds that changes in blood flow and vessel density in the eye may be an indicator of problems with blood circulation in the brain, signaling the risk of cognitive decline from Alzheimer’s disease and other forms of dementia. By bridging eye and brain research, BrightFocus grantees are pursuing a bold “what-if” science that could provide earlier detection and diagnosis of Alzheimer’s and other types of dementia.
Watch Dr. Kashani discuss recent research to diagnose vascular dementia through retinal scans.

Dr. Amir Kashani
2020
Blood vessel changes in the eye may reveal early dementia.
2021
First-ever driving test to predict Alzheimer’s disease.
Grantee Ganesh Babulal, PhD, develops a first-of-its-kind driving test that can identify Alzheimer’s disease before other symptoms appear. By evaluating driving behavior, the team detects subtle changes in cognition that might be missed by traditional testing.

A researcher from Dr. Babulal’s lab holds a device that collects data on driving behaviors in older adults that can serve as biomarkers of Alzheimer’s disease. Photo courtesy of Ganesh Babulal, PhD.
2021
Critical funding for treatment of traumatic brain injuries, which are associated with dementia later in life.
BrightFocus and the Medical Technology Enterprise Consortium jointly award $500,000 grants to researchers at Astrocyte Pharmaceuticals and a partnership between Mitochon Pharmaceuticals and the University of Kentucky for the study of neuroprotective therapeutics for the treatment of brain injuries, which could lower risk of subsequent Alzheimer’s and related dementias.
Potential therapies target traumatic brain injuries, which can lead to dementia.
2021
New Alzheimer’s blood biomarker discovered.
Led by grantee Thomas Karikari, PhD, a research team identifies a less frequently measured biomarker of tau in the blood, BD-tau, that positively correlates with neurodegeneration and cognitive impairment before symptoms begin. BD-tau outperforms existing blood tests used to detect Alzheimer’s-related neurodegeneration and could lead to earlier and more accurate Alzheimer’s diagnoses.

Dr. Thomas Karikari
2022
First-of-its-kind artificial intelligence model developed that can one day detect Alzheimer’s disease by reading a patient’s retina images.
A team of Hong Kong scientists led by grantee Carol Cheung, PhD, develops a faster way to screen for Alzheimer’s disease using an AI model that reads retina scans. This highly accurate method could enable people, who would receive their results immediately, to benefit from early intervention and treatment.
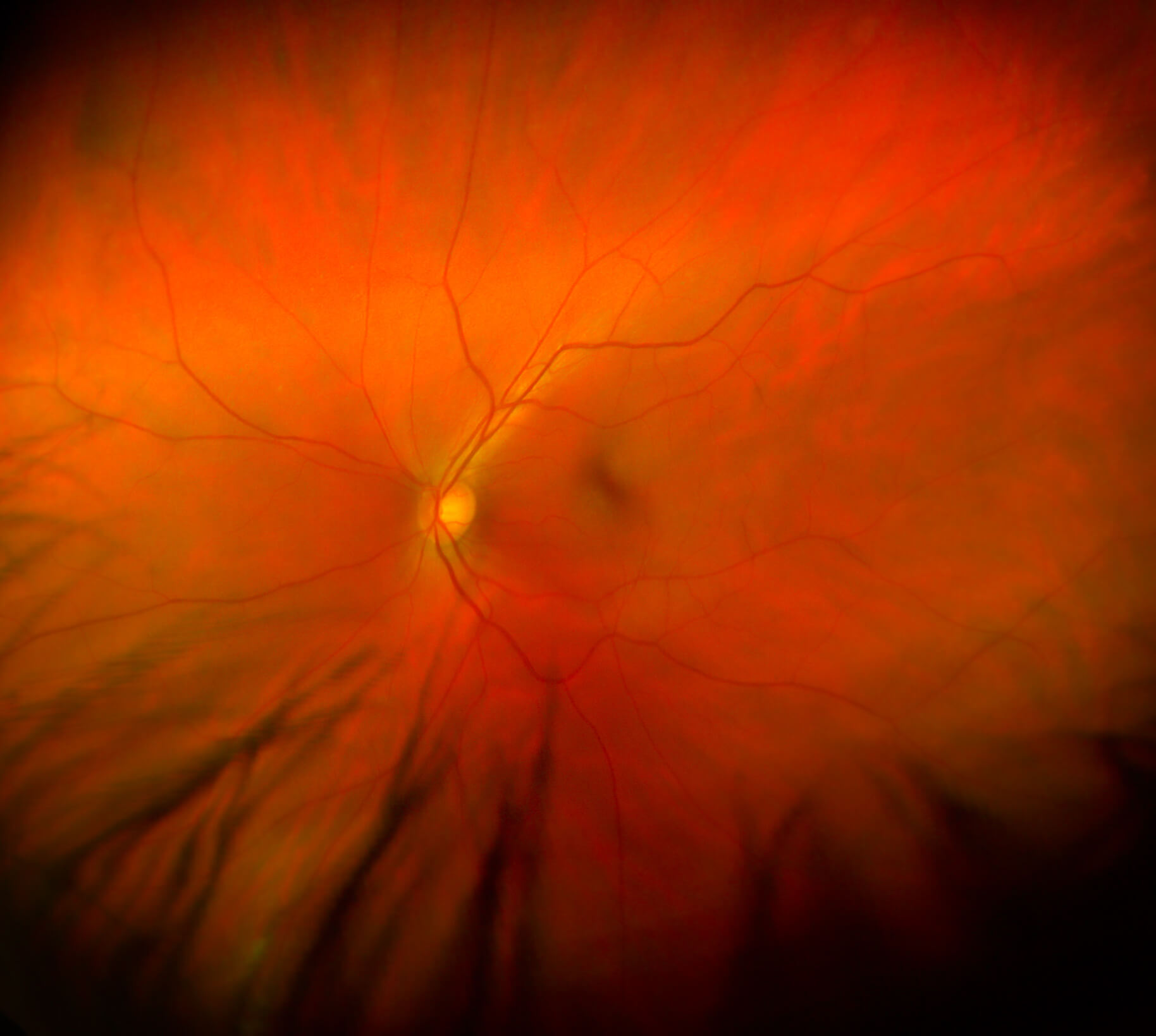
A close-up view of the retina, which could reveal critical signs of Alzheimer’s disease.