Drusen is a German word meaning “geode.” Since they are the defining feature of age-related macular degeneration (AMD), it’s important to understand what causes them to form. Understanding these mechanisms may lead to treatments that decrease the risk of disease progression.
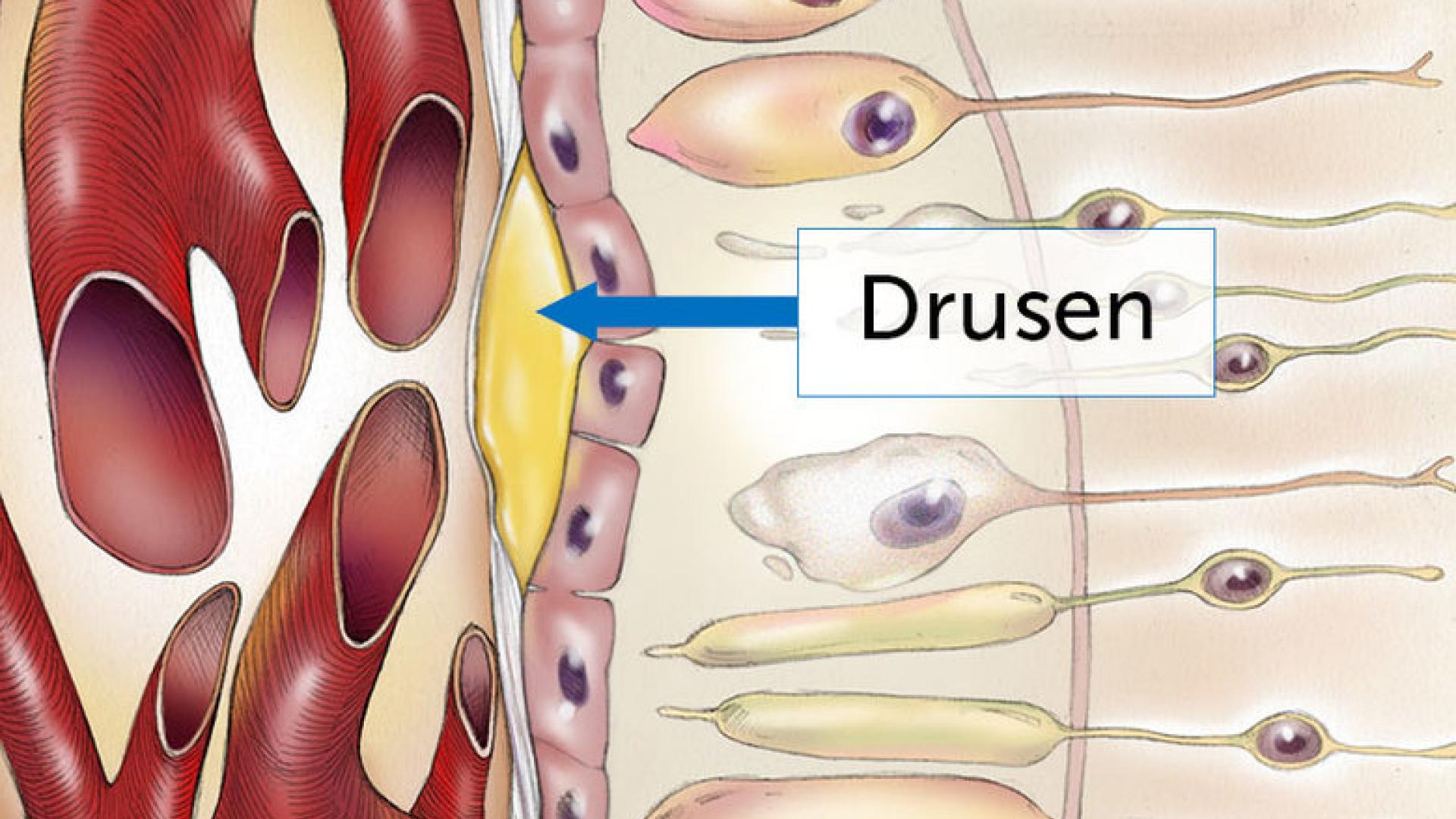
Drusen are the defining feature of AMD. They are tiny deposits under the retina that the eye doctor can see during an exam. They can also be photographed with a retina camera or using an imaging device called optical coherence tomography (OCT), which shows a cross-section of the retina and underlying drusen.
The Size and Number of Drusen are Important
Drusen are about the width of a pinhead and are composed of a mixture of proteins and lipids (naturally occurring molecules that include fats). They often cause no symptoms, but can occasionally cause visual distortion if they are very large and near the center of the retina.
The size and number of drusen are very important, as larger numbers of drusen, as well as drusen of larger size, indicate a higher risk for some vision loss in the future. Whereas the chance of a person with a few small drusen losing some central vision from AMD in a five-year timeframe is only a few percent, the chance may be as high as 50 percent over the same timeframe for certain people with many intermediate and large size drusen.
Drusen Contain a Complex Mixture of Proteins and Fats
To begin to discover why drusen form, researchers first investigated the contents of drusen. They are a complex mixture of proteins and fats. Several of the proteins are involved in immunity. These proteins normally function in a part of the immune system called the complement cascade, which can regulate white blood cells and poke holes in the membranes of invading bacteria. However, in patients with AMD, several complement proteins accumulate in drusen. The possibility that abnormal activity of the complement cascade is involved in AMD is strengthened by genetic studies showing that people with DNA sequence changes in several complement genes have an increased risk of AMD. An ongoing Phase II trial with a complement inhibitor made by Apellis has shown promise for the geographic atrophy form of AMD and is now enrolling patients in a Phase III trial.
Beta Amyloid
Another protein in drusen is beta-amyloid. This protein is also found outside of neurons in the brains of patients with Alzheimer’s disease, suggesting that the mechanisms of AMD and Alzheimer’s disease may be related. However, most patients with AMD do not have Alzheimer’s disease. So far, therapies aimed at preventing beta-amyloid formation or promoting the removal of amyloid beta in both AMD and Alzheimer’s disease have failed in clinical trials.
Cholesterol
Lipids, including certain forms of cholesterol, also accumulate in drusen, and genetic studies implicate lipids in AMD. A small study enrolling patients with very large drusen near the center of the retina showed that relatively high doses of a cholesterol-lowering statin drug were associated with drusen shrinkage and vision improvement. Further studies are needed before this approach can be widely recommended.
Cleaning Up Drusen
A study in mice showed that inhibiting the activity of a certain type of white blood cell, called macrophage, leads to the formation of drusen-like deposits in the retina. Since macrophages normally clean up unwanted debris or “garbage” outside of cells, the study suggests that macrophages clean up drusen before they accumulate to a clinically detectable size. Impaired macrophage function may contribute to drusen accumulation in patients.
Other studies have used cultured retinal pigment epithelial cells, the cells overlying drusen, grown in plastic dishes. Over weeks to months, these isolated cells excrete material that builds up underneath the cells and, in many ways, resembles drusen. This excreted material is probably some of the “garbage” that macrophages need to clean up to prevent drusen formation.
Mutated Enzymes
Another line of research has shown that patients with a rare form of hereditary drusen, as well as mice that model this disease, have a mutation in an enzyme that normally helps to maintain the normal structure of the membrane underneath the RPE cells, called Bruch’s membrane. So this indicates that proper maintenance of this membrane is important.
Drusen: Putting the Research Pieces Together
Taken together, these findings suggest that drusen form because RPE cells extrude (force out) unwanted or damaged proteins and lipids, which can then accumulate in Bruch’s membrane. Proteins from the complement cascade, along with minerals such as calcium, phosphate, zinc, and iron, then stick to this material, enlarging it and holding it together. Impaired macrophage function could contribute due to failure to clean up the “garbage.”
Whether the drusen are a cause or consequence of the disease process is unclear, but it seems likely that they are at least partly causal, as they can disrupt the flow of materials between the RPE and bloodstream, and they can distort the single layer of RPE cells. This may disrupt the blood-retina barrier function of the RPE. Consistent with this, a recent study from my laboratory suggests that eyes containing drusen leak blood components into the retina.
Since drusen are the defining feature of AMD, and their number and size are associated with the risk of vision loss from AMD, an increased understanding of drusen is likely to lead to new vision-saving drugs or treatments.
About BrightFocus Foundation
BrightFocus Foundation is a premier global nonprofit funder of research to defeat Alzheimer’s, macular degeneration, and glaucoma. Since its inception more than 50 years ago, BrightFocus and its flagship research programs—Alzheimer’s Disease Research, Macular Degeneration Research, and National Glaucoma Research—has awarded more than $300 million in research grants to scientists around the world, catalyzing thousands of scientific breakthroughs, life-enhancing treatments, and diagnostic tools. We also share the latest research findings, expert information, and resources to empower the millions impacted by these devastating diseases. Learn more at brightfocus.org.
Disclaimer: The information provided here is a public service of BrightFocus Foundation and is not intended to constitute medical advice. Please consult your physician for personalized medical, dietary, and/or exercise advice. Any medications or supplements should only be taken under medical supervision. BrightFocus Foundation does not endorse any medical products or therapies.
- Disease Biology