Can Stem Cells Help My Vision?
Featuring
Thomas V. Johnson, MD, PhD
Professor of Ophthalmology, Johns Hopkins Wilmer Eye Institute


Thomas V. Johnson, MD, PhD
Professor of Ophthalmology, Johns Hopkins Wilmer Eye Institute

This Glaucoma Chat episode featured Thomas V. Johnson, MD, PhD, who discussed the current research on neuroregeneration & neuroprotection using stem cells for glaucoma.
Download English Transcript PDF Download Spanish Transcript PDF
DR. DIANE BOVENKAMP: Hello, and welcome to the BrightFocus Glaucoma Chat. My name is Dr. Diane Bovenkamp, Vice President of Scientific Affairs at BrightFocus Foundation, and I am pleased to welcome you to the Chat today. BrightFocus Glaucoma Chats is a monthly program in partnership with the American Glaucoma Society and is designed to provide people living with glaucoma, and the family and friends that support them, with information provided by glaucoma experts. The American Glaucoma Society counts the leading glaucoma specialists in the country in their membership, and we are looking forward to hearing them discuss many topics about glaucoma during this Chat series.
Last month, we asked in the poll what listeners wish they had more information about, and 23 percent of listeners said research development. So, today we are going to talk about one research development that is so exciting—at least to me, and I hope it will be to you. The topic is “Can Stem Cells Help My Vision?”
BrightFocus funds exceptional scientific research worldwide to defeat Alzheimer’s disease, macular degeneration, and glaucoma and provides expert information on these diseases. You can find much more information on our website, www.BrightFocus.org.
Now, I’m pleased to introduce today’s guest, Dr. Thomas V. Johnson, who is a clinician scientist and the Shelley and Allan Holt Rising Professor of Ophthalmology at Johns Hopkins Wilmer Eye Institute. He completed his PhD in clinical neuroscience at the University of Cambridge in the United Kingdom, where his doctoral research involved directing a collaborative project between a stem cell laboratory at Cambridge and a molecular biology laboratory in the National Eye Institute’s NIH Intramural Research Program, studying stem cell transplantation as a potential neuroprotective treatment for glaucoma. And personally, it’s just such a pleasure to have you, Dr. Johnson, to join us today, and I’d like to welcome you for today’s BrightFocus Glaucoma Chat.
DR. THOMAS JOHNSON: Thank you so much, Diane. It’s really a pleasure to be here, and I just want to commend you and BrightFocus and the AGS for putting on these talks, which are a really fantastic way for patients and their families with these diseases, they can learn more about all the new and exciting research that is coming down the pipeline.
DR. DIANE BOVENKAMP: Great. And I know that you’re really, really good at explaining things in lay language, so I know that everybody’s going to be able to get a lot of information from you today. All right. So, let’s start with the basics. What are stem cells, where do they come from, and are all stem cells the same?
DR. THOMAS JOHNSON: Right. So, people have probably been hearing about stem cells for years now, and there’s been a lot of hype and excitement in the media and in the scientific field about the potential of stem cell therapy to cure all sorts of diseases. And the reason for that is that stem cells are a very unique and special type of cell. A cell is sort of the building block of anything that’s alive—plants, animals, humans are all made up of cells. And human beings contain trillions and trillions of cells that all have very specialized properties. We have skin cells and blood cells and bone cells and nerve cells, which are the types of cells that form the brain and the spinal cord and the retina in the back of the eye. Stem cells are what’s called an “undifferentiated” type of cell, meaning they’re not one of these mature types of cells that form tissue in the adult body; they’re rather, sort of, a cell that has the potential to form any type of cell if it’s given the right information about what it should turn into. Sometimes people think about this as a high-school student who is learning about the world, about English and science and history, and they could grow up to be anything they want to be—an astronaut, a doctor, a lawyer—but it’s not until they go through further training—they go to college or they go for on-the-job training—that they narrow down into a specific specialty. So, that’s the idea behind differentiation of cells.
Stem cells have two really important properties. One is that they are undifferentiated, but given the right instructions, they can form any type of cell in the human body. So, from a stem cell, we can make bone cells or blood cells or nerve cells. And then the second thing is that stem cells can proliferate, meaning they can divide to make two exact copies of themselves. And if you have one cell that makes two, then two can make four, and four can make eight, and these cells can divide exponentially forever. So, from a single stem cell, we could in theory make trillions and trillions of other stem cells to treat every patient on the planet. So, that’s where this excitement about stem cell therapy comes from.
Where do we get stem cells from? Well, for a long time the only way we could get stem cells was from an embryo. You may have heard of “human embryonic stem cells.” And that’s because when a sperm fertilizes an egg and forms a single cell, that single cell develops into a person, and so by definition, we know that that cell that a person came from has the ability to form all the tissues in the adult body. Now, obviously, there were considerable ethical issues around obtaining stem cells from embryos and logistical issues, but a really amazing advance came in 2007, when a scientist named Shinya Yamanaka in Japan figured out that we can take adult cells that are not stem cells from the human body and put them into a petri dish, and by giving them specialized instructions, we can actually convert them back into stem cells. These are called induced pluripotent stem cells, or iPSCs, and the technology in the last decade has come a long way. Dr. Yamanaka won the Nobel Prize for this in 2012, and today, we can just take a skin sample or a blood sample or even a urine sample from a patient—because there will be little tiny cells in the urine sample. We can take those, put them in a petri dish, and convert them into stem cells. So, we now have the technology to create stem cells from any patient in the world, pretty much at will.
DR. DIANE BOVENKAMP: Wow. So, this is scientists trying to almost replicate what nature already coded for, that there’s these cells that are in reserve, that there’s a special code of proteins and whatnot that make it go forward to turn into whatever cell it wants. Now everyone in the world can take a cell and dial it back to this common ancestor and then, if you have the right code you can turn it forward, right?
DR. THOMAS JOHNSON: Yeah. That’s exactly right. And the reason this is important is that there are a lot of conditions, injuries, diseases in the body where the body will heal itself. If you get a cut in your arm, then there are cells that will divide and fill in that cut and heal up the skin. If you break a bone, it can heal itself.
DR. DIANE BOVENKAMP: Exactly.
DR. THOMAS JOHNSON: But there are many diseases that can’t do that. Cardiovascular disease is one, but a really important class of diseases are neurodegenerative diseases, or diseases that affect the brain, the spinal cord, and the retina inside the eye. For whatever reason, mammals, which includes humans, have lost the ability to repair those neural tissues when they’ve been injured or when there’s neurodegenerative disease. So, the idea behind stem cell therapy for neurodegenerative disease is that we can take these stem cells, and if we can figure out how to turn them into the correct type of nerve cell that’s been lost in a particular patient, we might be able to replace those nerve cells and bring back the function that has been lost.
DR. DIANE BOVENKAMP: And it’s been difficult in the past because, if you think about spinal cord injury, the retina’s an extension of the brain, and so you need to find a way to get over scar tissue and whatever. I guess you can talk about that later; I’m just getting excited and ahead of myself here.
DR. THOMAS JOHNSON: Yeah.
DR. DIANE BOVENKAMP: So, I think another question that someone had was: Are there any conditions where the FDA has an approved stem cell–based treatment?
DR. THOMAS JOHNSON: Yeah, this gets to sort of what I was alluding to earlier—that we’ve been hearing about stem cells as a potential cure for disease for years and years and years, but there has been some disappointment by patients and others who are waiting for these treatments that they haven’t really hit primetime as far as being available in the clinic. There are some stem cell therapies that are available. So, for instance, when patients have different types of blood-borne cancers, they might get a bone marrow transplant. That’s something that’s been going on for decades. And that is a stem cell therapy. The cells that are being transplanted from the bone marrow are not iPSCs or human embryonic stem cells; they’re bone stem cells and blood stem cells that then take up residence in the patient’s bone marrow and can repopulate their own blood and immune system. So, that is an example of a stem cell therapy that’s been around for quite some time.
And then there are other cell-based therapies that are really becoming more and more popular and becoming increasingly approved to treat disease. Patients on the call may have heard of CAR-T therapy, which is a type of cell-based therapy where a patient’s T cells from their blood, which is part of their immune system, can be taken out of the body, manipulated in a culture dish in order to recognize a certain kind of cancer, and then injected back into the blood, where those cells will go and help fight the cancer. And there are also types of stem cell therapies where a patient’s own skin cells or cartilage cells can be harvested and forced to divide and repopulate in a dish and then transplanted back into the patient. And if those are skin fibroblasts, they might be used to treat burns or large wounds. Chondrocytes, which are cartilage stem cells, have been used to treat arthritis. So, there are new stem cell and cell-based therapies that are coming down the pipeline all the time.
At this point in time, there are not any FDA-approved treatments that use pluripotent stem cells, like the iPSCs that I was describing that have the ability to turn into any cell type in the body. But there are many in clinical trial, including for diseases of the central nervous system. There are stem cell–based therapies in clinical trial for Parkinson’s disease and Huntington’s disease and Alzheimer’s disease, and in fact, there are also clinical trials for eye diseases. For patients with age-related macular degeneration, you can take stem cells and turn them into retinal pigmented epithelium, and those are being transplanted underneath the retina into eyes of patients with macular degeneration, and very soon, there will be clinical trials where stem cells are used to create photoreceptors—the rods and cones in our eyes that detect light and turn it into a signal that the brain can recognize. So, pretty soon, there will be clinical trials in patients that have very poor vision because their rods and cones have degenerated, and the investigators involved in those trials will be transplanting stem cell–derived rods and cones back into patients’ eyes to see if that can regenerate vision that has been lost.
DR. DIANE BOVENKAMP: Yeah, and this is really, really exciting and encouraging for people and families affected by glaucoma because there’s already stem cell–based therapies that are out there already. We just don’t know it because it’s not called that; it’s called all these other things that you were talking about—CAR-T and bone marrow transplantation, etc.—and so, it’s really encouraging that we’re getting really close, and we just need to address the uniqueness of glaucoma.
DR. THOMAS JOHNSON: That’s absolutely right.
DR. DIANE BOVENKAMP: So, I guess that leads us to: How does glaucoma cause vision loss, and why, so far, is vision loss from glaucoma irreversible?
DR. THOMAS JOHNSON: Right. I think in order to understand that question, I’ll talk a little bit about the anatomy of the eye and the visual pathway. The listeners on the call may already be familiar with glaucoma in some terms, but I like to think of the eye sort of like a camera. At the front of the eye, you’ve got this clear window, the cornea, and then in the middle of the eye you have a lens. And those two things together help focus light onto the back of the eye wall. And at the very back of the eye is a neural tissue, the retina. You’ve described this perfectly before as sort of an outgrowth of the brain. So, that’s brain tissue at the back of the eye, and the lens and the cornea are meant to focus light coming into the eye onto the retina. And the retina really is like the film inside the camera. It is what the light hits and actually converts light entering the eye into a picture of the world around us that we all can see and appreciate.
Now, I’ve already talked a little bit about the retina and told you that it’s composed of some nerve cell types. We’ve talked about photoreceptors, which are the rods and cones, and those are cells that respond to light. When light hits them, they change their electrophysiological properties, meaning they are electrically active, and they will fire action potentials or have an electrical signal in them that corresponds to how much and what type of light is hitting those cells. But then, the retina begins to process that information before it sends it to the brain. So, the rods and cones will communicate that information to an intermediate type of neuron in the cell, called a bipolar cell. And then the bipolar cells, in turn, communicate that processed visual information to a very specific type of cell called the retinal ganglion cell, or RGC. Now, retinal ganglion cells are my favorite type of cell.
DR. THOMAS JOHNSON: They’re very, very interesting cells. They have cell bodies, which are sort of like their headquarters that live within the retina on the back wall of the eye, and there are, in humans, somewhere between 300,000 to 3 million retinal ganglion cells (RGCs) per eye speckled all over the retina. And those ganglion cells then have processes—little finger-like projections—that come out of the cell body in two directions. First, they have a type of projection called a dendrite, which is a little finger-like projection that grows towards the bipolar cells deeper in the retina and forms communication stations with the bipolar cells. Those are called synapses. And a synapse is what allows two nerve cells to talk to each other by sending electrical impulses through the bridge that connects those two cells.
So, on the one hand, you have the listening end of the RGCs through the dendrites so that they can receive information about light coming into the eye. And then, retinal ganglion cells have a very long fiber that is called the axon, and this fiber goes from wherever the cell body is in the retina through the most superficial layer of the retina, which is called the retinal nerve fiber layer, until it gets to the optic nerve head. And in glaucoma patients, this is what the doctor is looking at when you’re positioned at the slit lamp and they’re shining that super-bright light in your eye with a hand-held lens—they’re looking at your optic nerve head because that’s where all of the retinal ganglion cell axons from the eye are all coalescing in order to leave the back of the eye. Once those axons get out of the back of the eye, they form the optic nerve. And the optic nerve is the main connection between the eyeball and the brain. It’s how all the visual information from the eye gets to the brain so that a person can actually consciously perceive the vision that’s coming into that eye. And so, the optic nerve really is a fiber optic cable that’s composed of 300,000 to 3 million fibers from all of those RGCs, and it goes through the back of the eye socket into the brain.
It actually has sort of a complicated anatomy, where half of the fibers will actually cross to the other side of the brain, and half will stay on the ipsilateral—or same side—of the brain as the eye that they came from, but eventually all those fibers get into a part of the brain called the thalamus, which then sends those electrical stimulation signals that reflect vision to the brain, and that’s what allows people to see. So, with all of that, getting back to your original question: What is glaucoma, and why does it cause vision loss? So, glaucoma is a type of an eye disease called an optic neuropathy. And there are actually many different types of optic neuropathy. Glaucoma just happens to be the most common one; it’s the most prevalent optic neuropathy in the world. But all optic neuropathies are eye diseases in which the retinal ganglion cells, for whatever reason, die. In glaucoma, we’re still trying to figure out exactly what are the triggers and what it is that’s causing those RGCs to die, but we know that it has to do with the intraocular pressure—the pressure inside the eyeball—and how a person’s eyeball responds to that pressure. So, in patients with glaucoma, the pressure inside the eyeball is simply too high for the health of the nerve, and we think that may have to do with the fact that the eyeball is … you can think of it as a balloon. You know, if you blow air into a balloon, the wall of the balloon will expand and tighten up a little bit, and so pressure inside the eye also creates biomechanical stresses on the wall of the eye. And the optic nerve head, where all those RGC axons are leaving the eye, is sort of a weak point in the eyeball. And it may be that the pressure that builds up in the eye kinks those retinal ganglion cell fibers or somehow disrupts their ability to transport important proteins to and from the cell body. There may also be some aspects of this that deal with blood flow. And as the pressure in the eyeball builds up, then blood has a harder time getting into the optic nerve head to make those retinal ganglion cell fibers healthy. But at the end of the day, people with glaucoma have pressure that’s too high, and that causes retinal ganglion cell death and optic nerve degeneration.
As a glaucoma specialist, I can tell you—and I’m sure most of the listeners on this call know—that there are treatments for glaucoma. We can make the eye pressure lower using eye drops or lasers or sometimes operating room surgery. And when we make the eye pressure lower, that slows down the progression of the disease. It preserves people’s vision for longer periods of time. But nothing that we have available at this point in time is capable of restoring vision after it’s been lost. And that comes back to what I was discussing earlier, that the retina, just like the brain and the spinal cord, in human beings, is not naturally regenerated or repaired. So, when retinal ganglion cells die from glaucoma, they’re gone forever. And once they’re gone, even if the light is entering the eye and hitting the retina and stimulating photoreceptors and that information is communicated to bipolar cells, if there’s no RGC in that particular location of your visual field, your peripheral vision, then there’s no way for that information to reach the brain, and so people just are not able to see that part of their world around them.
DR. DIANE BOVENKAMP: Yeah, and that’s interesting because, really, we don’t see with our eyes; we really see and understand what we’re looking at with our brain. And I think that was really great, how you were describing all the connections between the different cells that this spark that’s created by vision has to travel. And if you think about it, like, as a 4×4 relay or maybe even a game of telephone or something, if one cell is passing off to another, but in a relay, if the RGC cells are hurt because they have a kink and they drop that baton, then they’re out of the race, right?
DR. THOMAS JOHNSON: Yeah, that’s exactly right.
DR. DIANE BOVENKAMP: And there’s no signal going through, right? So, yeah. I think that kind of goes to the next question, which is: How can stem cells treat glaucoma, and how could this be different from current approved treatments for glaucoma?
DR. THOMAS JOHNSON: In order to regenerate or restore vision that’s been lost in glaucoma, what we need to be able to do is actually take new RGCs and put them back where the patient’s own RGCs were before, with all their connections in the retina and the brain, restore those connections, and then it’s sort of, like, if you have a cable box and a TV, the cable box and the TV might be working okay, but if the cable connecting them to each other gets damaged, you need to replace the cable, and then everything will start working again.
DR. DIANE BOVENKAMP: Oh, that’s a great analogy.
DR. THOMAS JOHNSON: Yeah. So, it’s complicated, though, and this is one reason why some people on the call may be wondering why there’s clinical trials right now for AMD to use stem cells to replace cells that have been lost, why aren’t we there yet for glaucoma? And the answer to that question just lies in how much more complicated RGCs are than the other cell types that affect people with some of these other diseases. Photoreceptors are individual cells that don’t have any what we call “afferent synapses,” meaning they don’t have to connect to any other neuron to tell them what they need to know about vision because they’re responding to light directly. You know, the light is hitting the photoreceptors, and that’s telling them everything they need to know. Whereas retinal ganglion cells, if you just put them in the eye but they’re not connected to the bipolar cells in the retina, they won’t be receiving any of the information that they actually need to process and communicate on.
And then similarly, photoreceptors have one very short axonal projection that just goes a few micrometers within the retina and then connects directly to a bipolar cell right next to it, whereas, as I described, the RGCs have to grow this very long axon that goes through the optic nerve and into the brain. And in humans, that’s a matter of several centimeters. And then once the axons make their way into the brain, then they have to find the correct part of the brain, they have to grow into the correct region of the thalamus, and then even once they’re there, to expand on your analogy of telephone, you could think of the telephone switchboards that used to be in the basement of big office buildings in, like, the early 20th century. You have a million retinal ganglion cell fibers coming into the part of the brain that receives vision, and then there are going to be a million or more cells that have to receive that information from the RGCs. But those cells are arranged in a very precise pattern that mimics the topography or the arrangement of the scenery and the light and the visual world around you.
And so, in order for regenerated RGCs to provide people with useful vision, they not only need to get into and connect to the thalamus, but they need to do it in an arrangement that resembles the arrangement of those retinal ganglion cells within the retina. You know, if the RGCs connect within the brain in a haphazard fashion, a patient might see light versus dark, but the visual scene in front of them may just look like white noise or something, rather than an actual picture. So, where we stand now is trying to figure out how to get the retinal ganglion cells, after they’ve been transplanted into an eye, the ones that we have derived from stem cells, we need to figure out how to get those to then create the right synapses or connections with the neurons in the retina and the neurons in the brain.
DR. DIANE BOVENKAMP: Yeah, and dare I make it even more complicated, there isn’t just one RGC cell; there’s many different RGC cells, they have different functions, and it’s almost like, to connect up with the right part of the brain so you can see that, you know, you’re looking at a chicken and not a car, or something in front of you, then you have to have the correct, stop sign or green light to go this way or that way, almost like Hansel and Gretel crumbs that it has to follow through the brain to get to the right spot.
DR. THOMAS JOHNSON: Yeah.
DR. DIANE BOVENKAMP: And as you said, for binocular vision, you have to have some of it go on one side of the brain and some to the other, and yeah. It’s like a big challenge, a 3D puzzle, almost.
DR. THOMAS JOHNSON: Yeah. It’s very complex. And what I will say about this, though, is the idea of regenerating the visual pathway or replacing RGCs to bring back vision for patients with glaucoma has been around for decades. But years ago, most scientists, if you proposed this to them, would say, “This is science fiction. It’s so complicated that it’s never going to happen.” And I think what’s really changed in the last 10 years is we’ve made some really important advances in our understanding of stem cell biology and neurodevelopment that now point us towards believing that this really is possible, and it’s probably just a matter of time.
The two main developments that I want to point out are, first of all, the ability to manipulate and culture and harness the power of stem cells has improved exponentially over the last 10 or 15 years. So, when I started my PhD, if anyone was listening very closely, they may have heard that my PhD was on stem cell transplantation for neuroprotection. So, we weren’t actually trying to regenerate RGCs at that point, because in 2005, no one in the world had the ability to form an RGC in a dish from a stem cell. We didn’t know what instructions we needed to give stem cells in order to make them neurons, much less retinal ganglion cells. We now know what those instructions are, and in fact, there’s probably a dozen different related protocols that allow us in the laboratory to take a stem cell, expand it into billions of cells, and then over the course of about a month, turn them into retinal ganglion cells. So, we actually now have the tools available to us to begin the experiments to figure out what these cells are going to do when we transplant them into the eye.
The other thing is, as complicated as it sounds, that these cells—millions of cells—need to grow these fibers and find the correct location in the brain and then find the correct partner neurons to connect with, nature has shown us that there are very good ways of making this happen. Everyone can see when they’re born because this process occurs naturally during development, and it occurs very efficiently, and so part of what researchers have been doing over the last 10 years is studying different developmental systems to try to figure out, what are the signals that tell axons where to go, whether they need to cross to the other side of the brain or stay on their side of the brain? How do they end up in the lateral geniculate nucleus of the thalamus, which is the visual center of the brain, and not end up in the part of the brain that has to do with taste or smell, for instance? And so, we’ve now documented and figured out a lot of the signals that are present that tell these axons where they need to go when they’re trying to reach the brain.
And beyond that, we’ve figured out that there are fundamental differences between retinal ganglion cells when they’re newly born in a developing human or when that person becomes an adult. Neurons in general have much more plasticity during development, meaning they have the ability to grow, regrow processes, if they’re injured, they can make connections, they can withdraw their connections and make new connections. And then, as people mature, the connections and the ability of those neurons to change their configuration becomes drastically reduced. They are much less plastic. And some of the work that has really been seminal to the field of retinal ganglion cell regeneration has been figuring out: What are the signals within retinal ganglion cells that tell them, “You’re mature now, you should no longer be regrowing axons and making new connections”? And scientists have devised ways of either suppressing the inhibitory cues or activating pro-regenerative pathways within these cells that has allowed them, even in adult animals, to become much more regenerative.
And in mouse models of glaucoma and optic nerve injury, scientists have actually been able to take injured optic nerves where normally retinal ganglion cells would just die, and the few that survived would be stuck in the retina and never form connections to the brain. And by manipulating the inhibitory and pro-regenerative pathways, they’ve gotten—in adult mice—these RGC axons to regrow, all the way through the optic nerve, and in some cases to the correct parts of the brain that have helped those mice regain some level of visual function. So, I think we’re now at a point where the science is telling us this is something that is feasible. And now we just need to enhance or increase the efficiency of this process so that instead of regenerating a handful of retinal ganglion cells in a mouse, we want to be able to regenerate tens of thousands or hundreds of thousands of retinal ganglion cells in a human.
DR. DIANE BOVENKAMP: Yeah, just listening to you, I think it really strikes home to me. BrightFocus funds basic, translational, and clinical research, and it sounds like to be able to get the potential treatment through clinical trials and get them in the hands of the people who need them the most, you really need to have people working on all the different parts doing the basic research, as you said. Like, looking at, “Okay, what are the little Hansel and Gretel crumbs that we have to find out to try and move the pathway forward?” Dr. Yamanaka found a way to dial back the skin cells to pluripotent, and then someone else just found the code to get it forward to an RGC, and all the different types of RGCs. That’s, I guess, my plug for funding basic research, but I think I’m going to put a couple questions here together, again. One is, “Are there other eye diseases for which stem cell therapy is closer for reaching patients?” You already mentioned that it was for age-related macular degeneration. I know that there’s one in clinical trials. But I think focus on: Why is it taking longer to start clinical trials for glaucoma than age-related macular degeneration?
DR. THOMAS JOHNSON: Yeah, I think it comes back to the difference in the complexity in the type of cell that is lost or dies in the diseases that affect the outer retina, the photoreceptors—so, those are for patients with macular degeneration, retinitis pigmentosa, macular dystrophies—those cells are just inherently simpler cells with fewer connections, and the connections that they need to make to the retina are much closer and more orderly than retinal ganglion cells. Retinal ganglion cells have many more connections that are more complex, and so, figuring out the factors that we need to deliver to the eye in order to efficiently regenerate all those connections is a more complex task that’s just taking longer to fully understand.
DR. DIANE BOVENKAMP: Great. So, jumping off of that, you said there were a couple of things that got us to the point we are now. Is there a consensus in the glaucoma field for what remains to be studied before we can get to a point where this stem cell replacement therapy that truly connects the eye to the brain in this relay to recapitulate vision—so, if you see a butterfly in front of you, it looks like a butterfly—so that it can be taken to clinical trials?
DR. THOMAS JOHNSON: Yeah, I think up until recently, there hasn’t really been a consensus, and I want to go back to a point that you made that this field is very broad and very complex, so in order to try to regenerate RGCs in human patients, we are pulling from discoveries made by scientists in all sorts of different fields. Dr. Yamanaka probably had very little knowledge of optic nerves and retinas in general when he was working on stem cells, but that has turned out to be a really important avenue for the work that we’re doing now. And similarly, I think moving this work forward is going to require not just ophthalmologists but neuroscientists who understand how nerve cells make connections with each other; developmental biologists, who understand how the eye and the visual system develop naturally in humans; it’s going to require material scientists, who can develop scaffolds or specialized tools and devices that we can use to transplant retinal ganglion cells into the eye; it’s requiring optical engineers that can build very specialized microscopes to enable us to actually visualize what these transplanted neurons are doing inside the eye after they’ve been transplanted. And so, for quite some time, lots of different people have been working on small areas of this problem in isolation, with the idea that one day, we would put it all together. And so, a couple years ago, some colleagues and I founded a new consortium called the Retinal ganglion cell Repopulation, Stem cell Transplantation, and Optic nerve Regeneration Consortium, but for short, we call it RReSTORe, with two Rs.
DR. DIANE BOVENKAMP: Thank you for having an acronym for that.
DR. THOMAS JOHNSON: Yeah. Exactly. It started off as just a few dozen like-minded scientists who all were interested in the concept of retinal ganglion cell regeneration, and we wanted to have a conference to share ideas and see what the state of the field is and how we might work better together to help propel this field forward. And over the last 2 years, it’s actually grown now to include more than 220 investigators from all over the world, and as part of our work, we spent the last year and a half having both in-person and virtual discussions to try to hash out a very precise list of where the field stands now, what we know, what we don’t know, and what obstacles we think still remain in order to make retinal ganglion cell replacement a reality for patients with glaucoma and other optic nerve diseases. And so, we actually just finished completion of a white paper that should hopefully be published soon and available for anyone that’s interested to read that lays out all of the key experiments and areas of knowledge that we still need to gain over the next several years in order to bring this to a reality.
And then beyond that, we’re continuing to have ongoing collaborative discussions. I want to thank BrightFocus, who has contributed some funding to actually fund pilot projects among the scientists within the consortium to begin to answer some of these questions that we’ve identified, and we’ll be meeting again in person to catalogue our progress so far in April in Seattle before the meeting of the Association for Research in Vision and Ophthalmology.
DR. DIANE BOVENKAMP: Yeah, that’s perfect. It’s such a pleasure to support you, and yeah, I think I’ll make a note that … kudos on getting that white paper together. It’s going to be open access, and people can contact BrightFocus, we can always get you a copy of that white paper when it comes out. Another question that someone had was: What can I do if I’m interested in participating in stem cell research?
DR. THOMAS JOHNSON: So, I think it’s important to really understand, first of all, for any kind of clinical research, what the study is about, who is running it, what it’s based on as far as preliminary data regarding the safety and efficacy of the procedure, and knowing about the oversight of the research that’s being conducted. What I can say is at this point in time, I don’t know of any clinical trials of stem cell therapy that are being conducted in human patients for glaucoma or other optic nerve diseases. Now, that may change in the future—we think it will. But there are, for instance, stem cell transplantation clinical trials going on for macular degeneration. So, I’ll talk about this in general for both groups of people who may be interested in something that’s available today or that will be available hopefully in the coming years. And the reason I want to bring this up is because there is, shall we say, some “clinics”—and you can’t see me right now, but I’ll use air quotes around those “clinics”—
DR. DIANE BOVENKAMP: We can visualize that.
DR. THOMAS JOHNSON: Yeah, exactly. That exist throughout the world, and even some in the United States, that purport to be conducting clinical trials for stem cell research for eye disease. And those clinics may even register a clinical trial on the website ClinicalTrials.gov, which is a database that patients can go on and look at all the different clinical trials that may be going on throughout the world. But an important thing to understand about that website is that even though it’s hosted by the government, it’s not vetted in any kind of a way, and it will tell you right on the website that listing a trial on ClinicalTrials.gov does not imply that it’s been approved by the FDA or otherwise reviewed by anyone with regulatory power. And essentially, anybody can go on that website and register for a clinical trial, and it will go on the website. So, it’s important for patients to know, if they’re thinking about participating in a clinical trial, it should be registered on ClinicalTrials.gov, but that alone does not necessarily mean that it has been vetted or is safe or likely to be effective.
The other thing I’ll say about some of the quote-unquote “clinical trials” of stem cell therapy for eye disease is that, oftentimes, they have been charging patients significant amounts of money to participate in the trial—I’m talking about $15,000 to $30,000 to participate. And while it’s not unheard of for clinical trials to require some sort of fee for patients, it’s pretty rare. Usually clinical trials are supported by research grants or companies that are developing a therapy, and typically, it will be free for patients, and they may even be compensated for their time. But a red flag for any kind of a clinical trial would be that the trial requires a substantial sum of money from this participant to enroll. And lastly, most importantly, a handful of patients that have engaged in some of these supposed clinical trials where stem cells were being injected into the eye for treatments of glaucoma and macular degeneration and retinitis pigmentosa, those were based on very little, if any, preclinical data. They weren’t vetted to show that they would be safe. And tragically, there have been a series of case reports published in several journals, including the New England Journal of Medicine, that have documented when patients underwent those treatments, they actually ended up with worse conditions than they had started with. The stem cells proliferated inside their eye, they caused retinal detachments, and in some cases took away what little remaining vision was left.
So, what I want to say is, I totally understand that for diseases like glaucoma and macular degeneration, where sometimes patients may have lost a lot of vision or all of their vision, there can be desperation for anything that might be out there that can help them, and that’s exactly the motivation that drives all of the scientists doing this work. We’re all working as hard as we can to provide these treatments as soon as we can. But anyone interested in participating in research will want to make sure that what they are entering into is a fully vetted and interrogated and legitimate clinical trial. So, how can they do that? Well, first of all, not all clinical trials are associated with academic universities, but many of them are. And when clinical trials are taking place, they always need to be reviewed by an external, independent body of physicians and scientists called an Institutional Review Board, or IRB. And the job of the IRB is to make sure that the patients that are participating in these trials are kept safe and that the benefit to them exceeds any risk that may come to them by participating in the trial.
So, all of that is a long way to say that if patients are interested in participating in clinical research, I think that’s fantastic, that’s what’s needed in order to drive the field forward, but particularly in the field of stem cell transplantation, one just wants to make sure that they have all of the information possible available to them about what is being done as far as the procedure, what sort of data is it based on in laboratory experiments to give the participants confidence that this won’t cause problems, it will be safe, and that there’s at least a reasonable chance that it may help them. And they’ll want to make sure that it’s been thoroughly evaluated by an IRB.
DR. DIANE BOVENKAMP: Yeah, and it didn’t even occur to me that there wasn’t any vetting to go onto ClinicalTrials.gov, but I think it can be summed up as, “Trust, but verify.” So, what you can do is if you find something on ClinicalTrials.gov—or on our website we have a link to Antidote, where you can go and find a search—before you call that number, you can always print it out and bring it to your health care provider. Just ask them, “Is this a legit trial?” and they might be able to tell you some information.
DR. THOMAS JOHNSON: Yeah, I think that’s fantastic advice. I would always recommend discussing this with your ophthalmologist.
DR. DIANE BOVENKAMP: Yep. I personally heard some of the tragic stories about people going away to other countries and getting stuff put in their eye and then having … they lost their eye over it and everything, so just beware. So, here’s something that I think is exciting. Can you tell us about one of your current research projects and what’s most exciting about that research to you?
DR. THOMAS JOHNSON: Yeah, absolutely. So, when we started our work about 4 or 5 years ago, we had just gained the ability to take stem cells in a dish and turn them into retinal ganglion cells. And so, we began by transplanting those retinal ganglion cells into the eyes of mice that had glaucoma, hoping that those retinal ganglion cells would integrate into the visual pathway and replace the RGCs that had been lost. And to our dismay, what we found from the preliminary experiments was that the retinal ganglion cells would, for the most part, survive inside the eyeball, but they grew on the surface of the retina, and they did not seem to recognize that there was neural tissue just underneath them that they were supposed to grow dendrites into so that they could make those synaptic connections with bipolar cells and receive information about light.
So, we spent a couple years trying to figure out: What was that obstacle or that blockade that existed on the surface of the retina that prevented RGCs from actually integrating into the recipient’s retinal tissue. And to make a long story short, we found that there is an anatomic membrane that exists on the surface of the retina called the internal limiting membrane, or ILM, and this is a membrane that’s very important during development because it signals to the neurons in the retina which side of the retina is which and where they’re supposed to grow their dendrites in order to create a very nice, layered structure. But in adulthood, the ILM is totally dispensable, and in fact, there are classes of diseases, like macular holes and vitreomacular traction, where adhesions between the vitreous jelly in the center of the eye and the internal limiting membrane cause traction on the retina, which can cause distortions or even tears in the retina. And the treatment for that is vitreoretinal surgeons will do a surgery where they vacuum out the jelly in the eye, and then with very fine tweezers, they peel the ILM off the surface of the eye.
So, we figured if the ILM is not necessary in adult humans, then maybe we can just remove it or permeabilize it, and maybe that will help retinal ganglion cells integrate into the retina. So, we developed a couple of different ways to do this—one with transgenic mice, and one with just an enzyme that you could inject into the eyeball, and it would digest the ILM and chew little holes in the ILM so that it looked sort of like Swiss cheese, without damaging the retina underneath it. And then, when we went back and transplanted human stem cell–derived RGCs into those mice, we found that, lo and behold, they now were able to migrate into the retina and grow dendrites into the part of the retina where they could form those communication stations, or synapses, with bipolar cells.
So, right now, what we’re doing is trying to figure out how much the connections between donor RGCs and the host bipolar cells actually resemble the same connections that would’ve existed for the mouse’s own healthy RGCs. So, we’re doing what’s called electrophysiology, where we are measuring the electrical properties of the transplanted RGCs in real time with microscopes that detect fluorescent molecules. And we have molecules that will flicker in a fluorescent movie, depending on how active that retinal ganglion cell is. And we can do things like flash light at the retina, which the photoreceptors will recognize, and then communicate information about that flash to the bipolar cells, and we’re checking now to see if that information is then communicated to our donor RGCs in such a way that they’re receiving the input that they need about vision in order to communicate that to the brain. So, I think we’ve made a lot of progress on figuring out how to get transplanted RGCs to communicate with the retina in the way that they need to in order to restore that side of vision. And then the next major obstacle will be getting the axons from those donor RGCs to grow through the optic nerve and into the brain.
DR. DIANE BOVENKAMP: Wow. I mean, thank you so much for … you’re still in the beginning of your research career, you’re so distinguished, and you’ve already helped solve one of the major, major issues to move forward, so it’s just such a pleasure and an honor to chat with you. I know I could go on for another hour, but our time is, sadly, done. So, for everybody who had questions, we’ll try and get answers to them later. To close out today, Dr. Johnson, thank you so much for providing us with so much important information. I know I’m going to go back and maybe reread the transcript again. You’ve totally taken us to school. That’s like a master class on stem cells. So, before we conclude, are there any final remarks you’d like to share with the audience?
DR. THOMAS JOHNSON: No, thank you very much for hosting me. It’s been a real fun discussion, and I hope that it was informative for all the listeners.
DR. DIANE BOVENKAMP: Great, great. Thank you so much, and this concludes the BrightFocus Chat about glaucoma. Bye.
BrightFocus Foundation is a premier global nonprofit funder of research to defeat Alzheimer’s, macular degeneration, and glaucoma. Since its inception more than 50 years ago, BrightFocus and its flagship research programs—Alzheimer’s Disease Research, Macular Degeneration Research, and National Glaucoma Research—has awarded more than $300 million in research grants to scientists around the world, catalyzing thousands of scientific breakthroughs, life-enhancing treatments, and diagnostic tools. We also share the latest research findings, expert information, and resources to empower the millions impacted by these devastating diseases. Learn more at brightfocus.org.
Disclaimer: The information provided here is a public service of BrightFocus Foundation and is not intended to constitute medical advice. Please consult your physician for personalized medical, dietary, and/or exercise advice. Any medications or supplements should only be taken under medical supervision. BrightFocus Foundation does not endorse any medical products or therapies.
In clinical trials, stem cell therapy for glaucoma shows promise for rebuilding the eye’s drainage system and protecting the optic nerve.

In this chat, Dr. Poonam Misra addresses some of the most common questions listeners have shared over the past year—from treatment options and lifestyle considerations to the latest educational resources.

Dr. Astrid Werner explains what causes dry eye, how to recognize the symptoms, and effective treatment options—including preservative-free drops, artificial tears, and eyelid care routines.
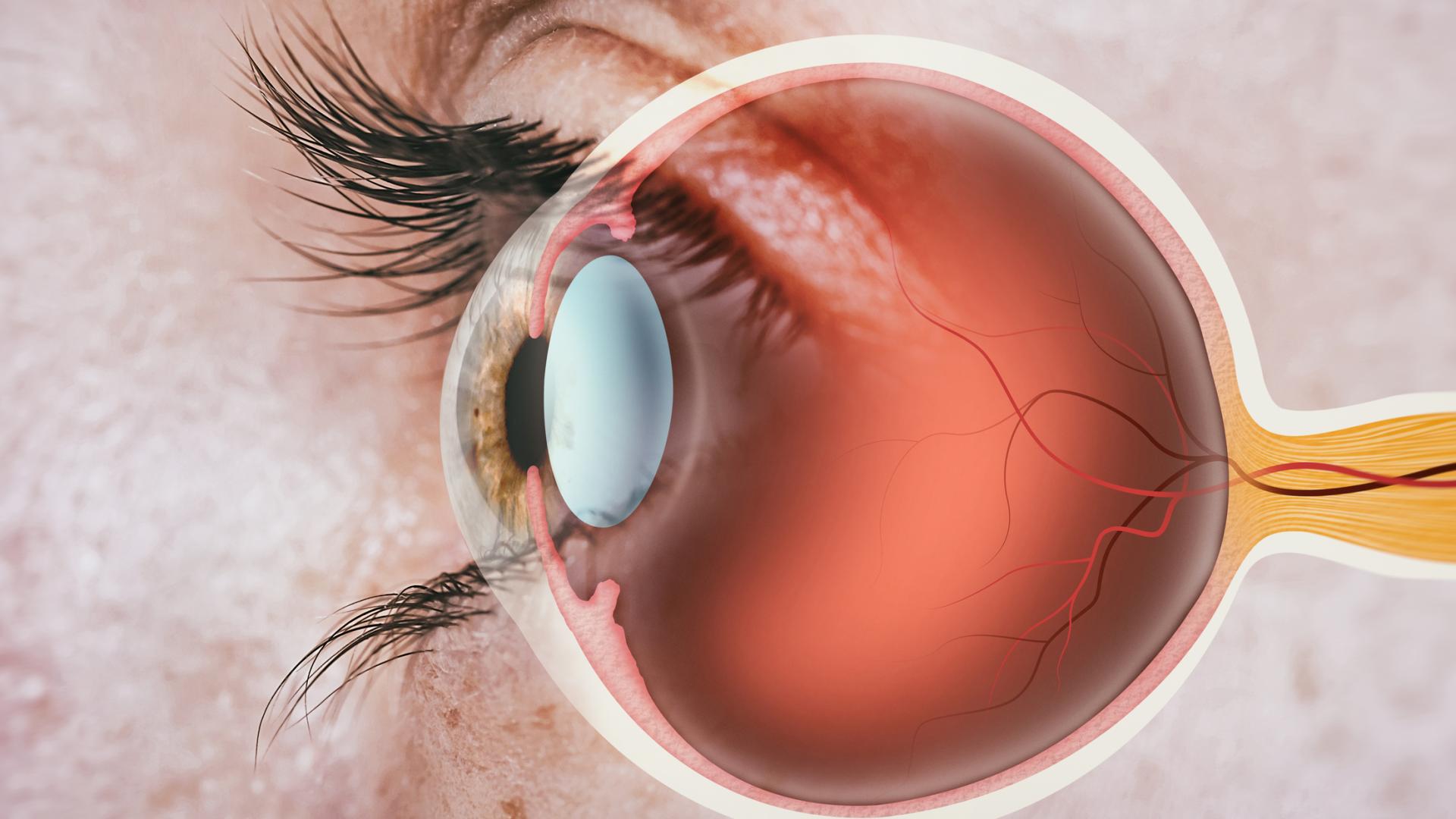
Join us for a fascinating conversation with Dr. Lucy Q. Shen as we explore cutting-edge research into restoring vision loss from glaucoma.
Support Groundbreaking Glaucoma Research
Your support helps fund critical research that could prevent vision loss, provide valuable information to the public, and cure this sight-stealing disease.
Donate Today