Promising Research Looks to Clinical Uses of IL-18 to Prevent Wet Age-Related Macular Degeneration (AMD)
Written By: BrightFocus Editorial Staff
Written By: BrightFocus Editorial Staff
This research was supported by BrightFocus Foundation.
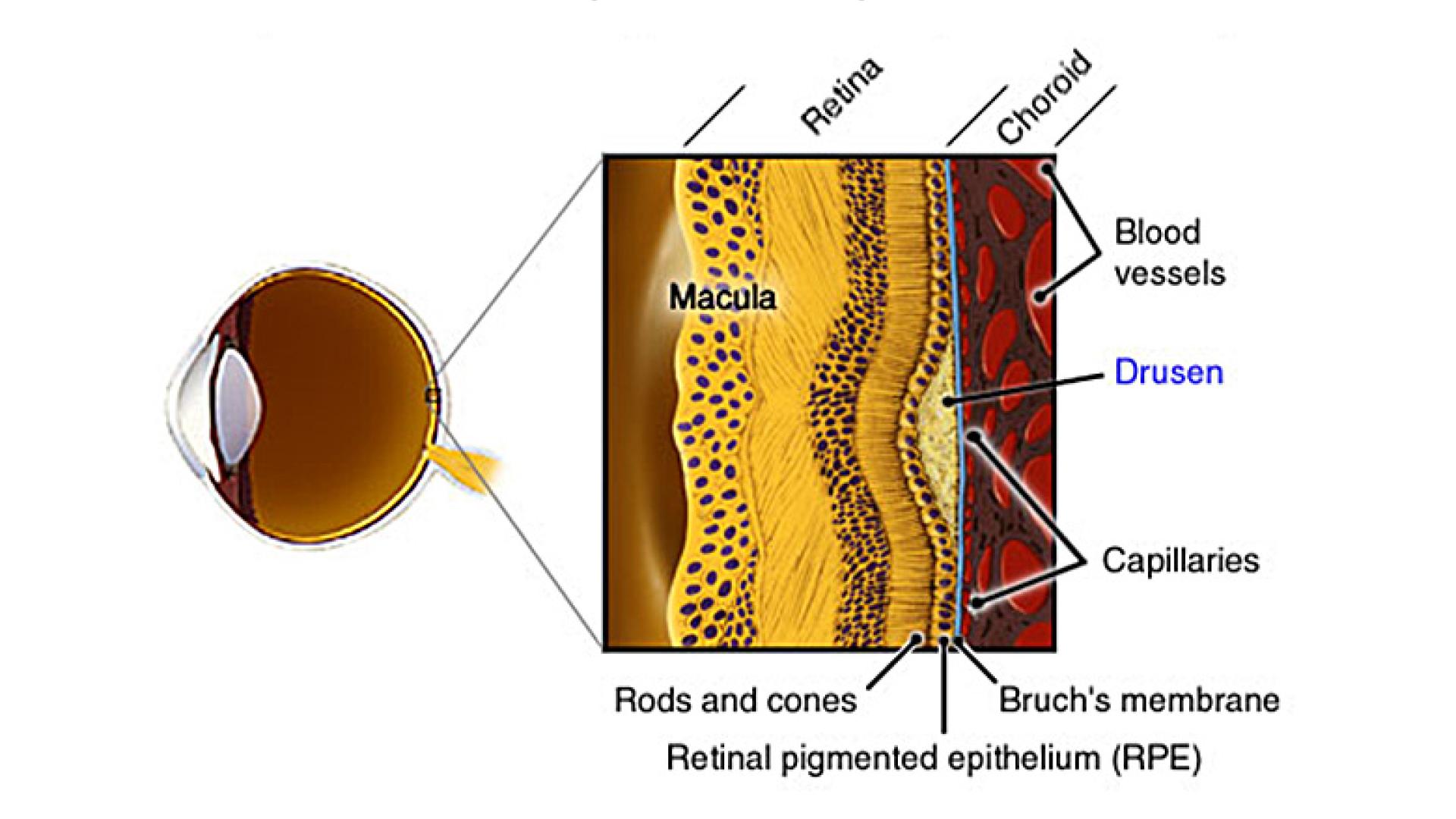
In an online report published April 2, 2014, BrightFocus researchers Sarah Doyle, PhD, Matthew Campbell, PhD, and Peter Humphries, PhD, and their teams, have reported from studies in mice that the inflammatory cytokine interleukin-18 (IL-18) can prevent choroidal neovascularization (CNV) formation—the fragile, leaky blood vessels forming on the retina that are the hallmark of wet AMD—and is not toxic to the retinal pigment epithelium (Doyle et al, Sci Trans Med, 2014).
Earlier research has suggested that the immune system plays a central role in the development of both “dry” and neovascular (“wet”) forms of macular degeneration. Many investigators, including several funded by BrightFocus Foundation, have dedicated themselves to differentiating which genetic switches might turn the immune system on–and whether the resulting inflammation might be harmful to or protective of the retina. It’s a continuing scientific conundrum and controversy, and there is evidence pointing both ways.
These latest findings move Doyle and colleagues further down one side of this two-way hypothesis. Their findings also support the working hypothesis that IL-18 may represent a potential future treatment to slow or stop progression from dry AMD to wet AMD.
It’s the second breakthrough in two years for Doyle et al since she and Campbell, working in the lab of Peter Humphries, PhD, made the discovery that drusen isolated from donor AMD eyes can trigger an immune response by a collection of proteins known as an “inflammasome,” leading to abnormal growth of blood vessels and wet AMD. Also, in that 2012 paper, they hypothesized that one of the inflammasome proteins, IL-18, has a possible protective effect (Doyle S et al, Nature Medicine, 2012).
“We feel it is important to acknowledge the contribution of BrightFocus Foundation,” Doyle said in a letter after this latest paper had been accepted for publication. “[Both of] our labs have been funded in part by your organization throughout the duration of the study.”
In another study, a team of researchers, including BrightFocus-funded Dr. Judit Baffi, identified three “guilty parties” that, in mice, can trigger the death of retinal pigmented epithelial (RPE) cells, which leads to blindness. It found that these three culprits—including IL-18—could be prevented from killing the RPE cells by using drugs or genetic techniques to block their activity (Tarallo V et al, Cell, 2012). Doyle and colleagues addressed the role of RPE in their new paper, noting that administration of IL-18 had no effect on RPE cell viability, but that over-expression of IL-18 precursors alone can cause RPE cell swelling and subsequent atrophy, a process that can be inhibited by the promotion of autophagy. In other words, it may be that a processed form of IL-18 is beneficial, but either the unprocessed form of IL-18, or too much of any form of IL-18, can take the retina down the path towards RPE atrophy and corresponding angiogenesis, leading to wet AMD—but at this point, it’s only speculative.
While clinical trials are being discussed based on Doyle and colleagues’ findings, any treatments are still years off. Yet, in a preliminary test of IL-18’s potential therapeutic usefulness, Doyle and Campbell were able to show that in mice manipulated to exhibit early AMD, systemic delivery of processed IL-18 is effective alone, yet even more effective in combination with the current gold standard of treatments, eye injections of anti-VEGF therapy. The idea of earlier intervention and a possible combination therapy for intermediate to advanced AMD is extremely promising. AMD is a highly individualized disease that progresses and responds to therapy differently based on the individual. To be able to “customize therapy” would be very helpful indeed.
In earlier BrightFocus-funded research, these researchers traced activation of the inflammasome by drusen to the development of dry AMD. At the time they hypothesized that drusen might provide a therapeutic target to arrest the disease (Doyle S et al, Nature Medicine, 2012).
The power of science is huge—but there are no shortcuts on the route to finding a cure for a disease as complicated as macular degeneration. The longest, and most difficult, research tends to take place in the beginning, when one discovery can take researchers down a long and winding road to another discovery—or to a dead end.
“Today, we have only once class of drugs against AMD,” commented Guy Eakin, PhD, vice president of Scientific Affairs at BrightFocus Foundation. “While these existing drugs have saved the eyesight of millions, more can be done to bring forward new classes of drugs. These results are giving us a better understanding of how the immune system might be managed to reduce the loss of vision due to AMD.”
Recognizing this, BrightFocus has funded both sides of this scientific debate over whether IL-18, as an inflammatory mediator, signals retinal preservation or retinal decline. Ideally, in the years and months ahead, more than one investigative pathway may converge in a central explanation of how macular degeneration works (ie, the “disease mechanism”). If that were to happen, it would advance knowledge—as well as opportunities for prevention or treatment—by a quantum leap. That ideal scenario—the scientific equivalent of “all roads lead to the same place”—cannot yet be ruled out with respect to AMD. The field is moving forward to that understanding everyday as researchers uncover the various triggers and manipulate the immune system to preserve retinal function.
“It’s a high-risk, high-reward business,” Eakin said. “We at BrightFocus are grateful for the opportunity, through our basic research grants, to advance scientific exploration and early discoveries on the road to a cure for macular degeneration—all thanks to the generosity of our donors.”
BrightFocus Foundation is a premier global nonprofit funder of research to defeat Alzheimer’s, macular degeneration, and glaucoma. Since its inception more than 50 years ago, BrightFocus and its flagship research programs—Alzheimer’s Disease Research, Macular Degeneration Research, and National Glaucoma Research—has awarded more than $300 million in research grants to scientists around the world, catalyzing thousands of scientific breakthroughs, life-enhancing treatments, and diagnostic tools. We also share the latest research findings, expert information, and resources to empower the millions impacted by these devastating diseases. Learn more at brightfocus.org.
Disclaimer: The information provided here is a public service of BrightFocus Foundation and is not intended to constitute medical advice. Please consult your physician for personalized medical, dietary, and/or exercise advice. Any medications or supplements should only be taken under medical supervision. BrightFocus Foundation does not endorse any medical products or therapies.
Help Fight Macular Degeneration and Save Sight
Your donation helps fund critical research to bring us closer to a cure for this sight-stealing disease and provide vital information to the public.
Donate Today