Tau Toxicity – New Insights from Down Syndrome-Related Alzheimer’s Disease
Written By: BrightFocus Editorial Staff
Written By: BrightFocus Editorial Staff
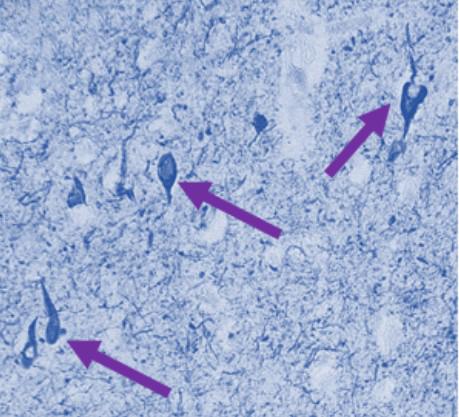
What: Extracellular vesicles derived from the blood of people with Down syndrome and Alzheimer’s disease (AD) can spread tau misfolding in normal mice.
Where: Ledreux et al, Small Neuron-Derived Extracellular Vesicles from Individuals with Down Syndrome Propagate Tau Pathology in the Wildtype Mouse Brain, Journal of Clinical Medicine, 2021.
BrightFocus Connection: The senior author of this article, Ann-Charlotte Granholm-Bentley, DDS, PhD, received a 2018 BrightFocus Alzheimer’s Disease Research (ADR) grant to develop the International Brain Bank for Down Syndrome-Related Alzheimer’s Disease.
Why It Is Important: Tangles of aggregated tau, a neuronal protein, have long been recognized as a sign of neurodegeneration due to Alzheimer’s disease (AD). These tangles can spread from one part of the brain to another, but researchers are still not sure exactly how. One theory contends that they spread through extracellular vesicles – small, membrane-enclosed sacs that ferry proteins, fats, and other molecules between cells. All neural cell types release extracellular vesicles into the surrounding environment which can then be taken up by neighboring cells or cleared from the brain through the cerebrospinal fluid and into the blood. It has recently been shown that such vesicles isolated from the plasma of people with Alzheimer’s can spread the disease by transmitting toxic proteins like tau between neuronal cells.
People with Down syndrome develop Alzheimer’s disease at much higher rates than the general population because they have an extra copy of chromosome 21 which holds the amyloid precursor protein gene. Dr. Granholm has been studying neurodegenerative diseases, including Down syndrome and AD, for decades, with a more recent focus on the trafficking of extracellular vesicles. This study sought to determine if neuron-derived extracellular vesicles isolated from the blood of people with Down syndrome-related AD can seed toxic tau species in the brains of normal mice and if so, which cells are responsible for mediating the uptake and transmission of toxic tau.
Extracellular vesicles were injected into the hippocampus of old mice. The hippocampus is a brain region important for learning and memory and compromised in normal aging and AD. Two different types of tau species were analyzed in the sub-regions of the hippocampus and in neighboring brain regions. Aggregated tau protein was observed around the injection site one month after injection of extracellular vesicles. By four months post-injection, tau spread throughout the hippocampal sub-regions and into the corpus callosum (the bundle of nerves that connects the left and right hemispheres of the brain, allowing them to communicate with one another), indicating efficient transfer of toxic protein between cells. Both tau species were predominantly localized to neurons, although the regional distribution varied, and one species was also observed less frequently in glial cells. Extracellular vesicles derived from people with Down syndrome and AD also elicited a greater inflammatory response that was evidenced by increased numbers of reactive microglia in the hippocampus.
The researchers conclude that extracellular vesicles isolated from people with Down syndrome contain toxic human tau species that elicit aggregation and propagation of endogenous mouse tau, as well as a neuroinflammatory response in normal mouse brains. . As a next step, the researchers intend to determine other components within exosomes that may drive the pathological responses observed. They note that FDA-approved drugs in the cancer field can potentially be repurposed to target the creation, release, and uptake of these vesicles, which may be a promising treatment approach in the future to prevent AD from spreading.
BrightFocus Foundation is a premier global nonprofit funder of research to defeat Alzheimer’s, macular degeneration, and glaucoma. Since its inception more than 50 years ago, BrightFocus and its flagship research programs—Alzheimer’s Disease Research, Macular Degeneration Research, and National Glaucoma Research—has awarded more than $300 million in research grants to scientists around the world, catalyzing thousands of scientific breakthroughs, life-enhancing treatments, and diagnostic tools. We also share the latest research findings, expert information, and resources to empower the millions impacted by these devastating diseases. Learn more at brightfocus.org.
Disclaimer: The information provided here is a public service of BrightFocus Foundation and is not intended to constitute medical advice. Please consult your physician for personalized medical, dietary, and/or exercise advice. Any medications or supplements should only be taken under medical supervision. BrightFocus Foundation does not endorse any medical products or therapies.

University of Colorado
Every Donation is a Step Forward in the Fight Against Alzheimer’s
Your donation powers cutting-edge research and helps scientists explore new treatments. Help bring us closer to a cure and provide valuable information to the public.
Donate Today