Explore key milestones and see how our researchers are improving lives.
1995
Researchers lay the groundwork for a better understanding of age-related macular degeneration—a disease that leads to loss of central vision.
Renowned English ophthalmologist Alan C. Bird, a member of the BrightFocus Scientific Review Committee, and his team, coin the terms “early and late age-related macular degeneration” and “dry and wet late age-related macular degeneration,” creating an international classification and grading system for age-related maculopathy and age-related macular degeneration.
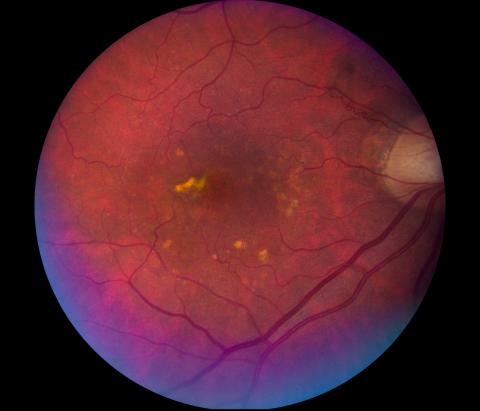
Image of a retina with dry age-related macular degeneration.
1995
Researchers lay the groundwork for a better understanding of age-related macular degeneration—a disease that leads to loss of central vision.
Alan C. Bird, MD
Emeritus Professor and Consultant at the Institute of Ophthalmology at the Moorfields Eye Hospital, London
Best known for his work on retinitis pigmentosa and research into inherited retinal degeneration, Dr. Bird studied neurology and neurosurgery, but later turned to ophthalmology. While at the Institute of Ophthalmology, he worked with numerous fellows in a variety of multidisciplinary activities involving electrophysiology, specialized imaging, psychophysics, immunology, and pathology—which resulted in the development of new technologies to define the clinical characteristics of retinal disease. His studies have also correlated abnormal gene expression with metabolic dysfunction at the cellular level, which has led to a clearer understanding of retinal degenerative diseases, and has had significant implications for clinical management of these disorders, including better genetic counseling for patients and the development of new treatment approaches, including gene therapy. He has received many awards, including the Duke Elder, the Doyne and Bowman medals, the Prix Chauvin, and the Helen Keller Prize for Vision Research. Dr. Bird has undertaken extensive international work in Africa, tackling river blindness, and in Jamaica, examining the retinal changes that occur in patients with sickle cell disease.
1999
Macular Degeneration Research, a program of BrightFocus, begins.
2005
First anti-VEGF treatment for wet age-related macular degeneration.
Macular Degeneration Research grantee Peter Campochiaro, MD, is among the first to prove the benefits of suppressing vascular endothelial growth factor (VEGF), leading to the development of a novel anti-VEGF age-related macular degeneration treatment method that’s still the gold standard for helping to slow or stop further vision loss in wet age-related macular degeneration patients.
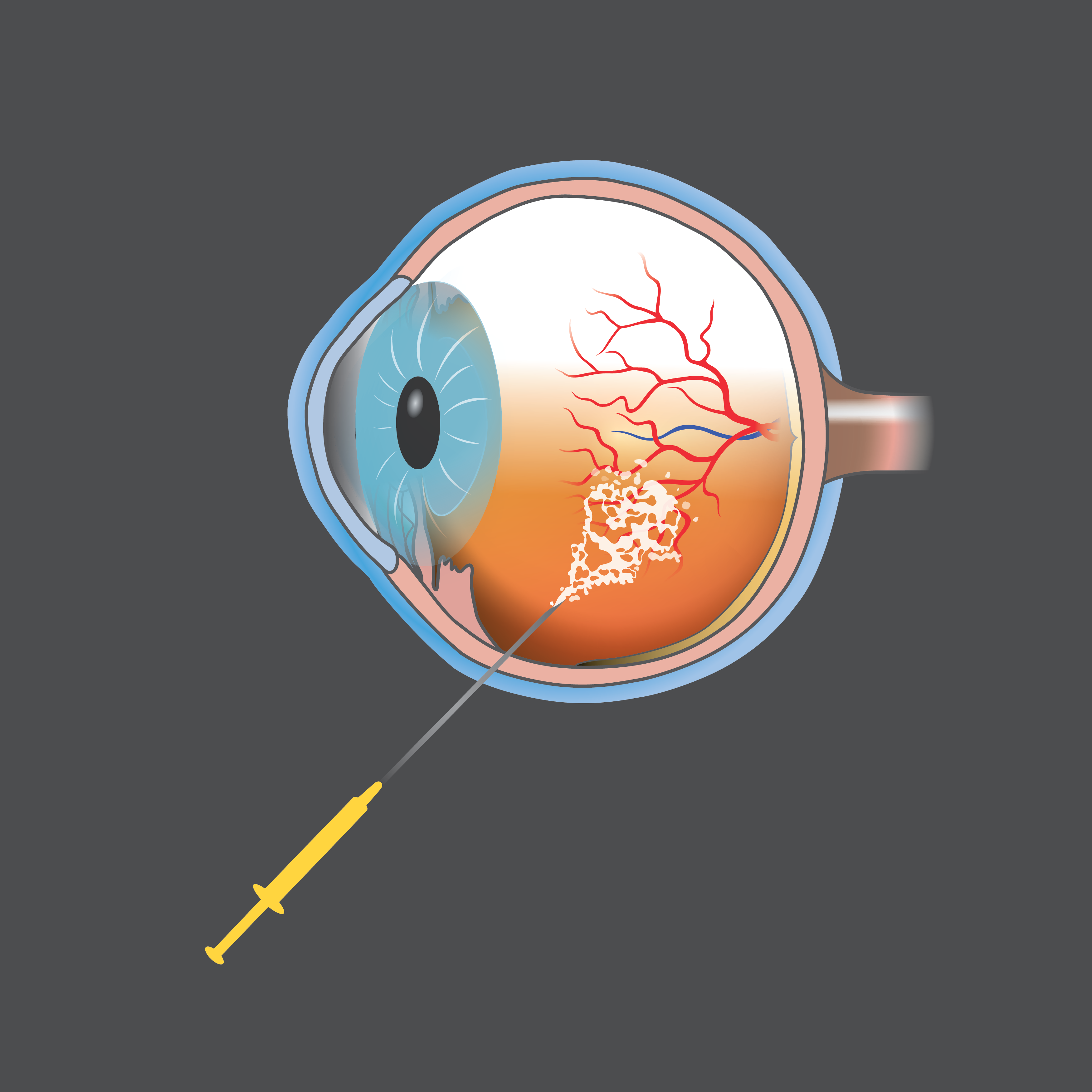
2006
Researchers discover the “complement pathway” in age-related macular degeneration—central to the development of the first and only treatment for geographic atrophy, a leading cause of blindness.
Research funded by Macular Degeneration Research helped lay the foundation for the discovery and understanding of the complement pathway, part of the immune system, and its involvement in onset of age-related macular degeneration.

2014
A new inflammatory marker discovered for wet age-related macular degeneration.
Macular Degeneration Research grantees Sarah Doyle, PhD, Matthew Campbell, PhD, and others in Dublin, Ireland, discover that the inflammatory marker IL-18 could be used to prevent wet AMD and lead to a better understanding of how the immune system could be managed to reduce vision loss.

Sarah Doyle, PhD, and Matthew Campbell, PhD
2014
A new inflammatory marker discovered for wet age-related macular degeneration.
2015
Advances in gene-editing technology improve the study and treatment of age-related macular degeneration.
BrightFocus-funded researchers, including Don Zack, MD, PhD, expand CRISPR gene-editing technology and develop a modified CRISPR technique that improves the speed and efficiency of gene function studies, aids in the development of new cellular models of diseases, and eventually could help treat genetic conditions.

A 3D rendering of the treatment and adjustment of DNA.
2021
A team of medical researchers and bioengineers, including grantee Ruchira Singh, PhD, develop the first-ever 3D cell matrix model of the human eye that replicates wet age-related macular degeneration.
The breakthrough discovery can help pinpoint what causes the disease, develop drug treatments, and determine how well drugs could work in specific patients, offering a more personalized approach to treatment.

Dr. Ruchira Singh (left) and colleague in the lab.
2021
Pioneering work by grantee Ilyas Washington, PhD, leads to FDA “Breakthrough Therapy” designation for potential Stargardt disease treatment, with possibilities for treating dry AMD—two vision diseases that can lead to blindness.
BrightFocus awarded Dr. Washington one of the first research grants of his scientific career, allowing him to begin exploring a possible role for enriched vitamin A in treating vision disease.

Dr. Ilyas Washington accepts the 2022 Bench to Bedside Award at BrightFocus’ gala.
2021
Pioneering work by grantee Ilyas Washington, PhD, leads to FDA “Breakthrough Therapy” designation for potential Stargardt disease treatment, with possibilities for treating dry AMD—two vision diseases that can lead to blindness.
“BrightFocus was the first major funder of my academic lab. They gave me the opportunity – and the confidence – to believe that I could someday stop someone from losing their sight.” – Dr. llyas Washington
2022
Historic first patient-derived stem cell therapy for dry age-related macular degeneration.
Macular Degeneration Research grantees Kapil Bharti, PhD, and Amir Kashani, MD, PhD, were part of the National Institutes of Health clinical trial team that pioneered the first patient-derived stem cell therapy for dry age-related macular degeneration. Our early research helped pave the way for this groundbreaking surgery.
Dr. Kapil Bharti (left) and Dr. Amir Kashani
2023
FDA approves first treatment for geographic atrophy, an advanced form of age-related macular degeneration.
Key early Macular Degeneration Research-funded findings from 2006 would be pivotal for the first FDA-approved complement pathway drug to treat geographic atrophy.
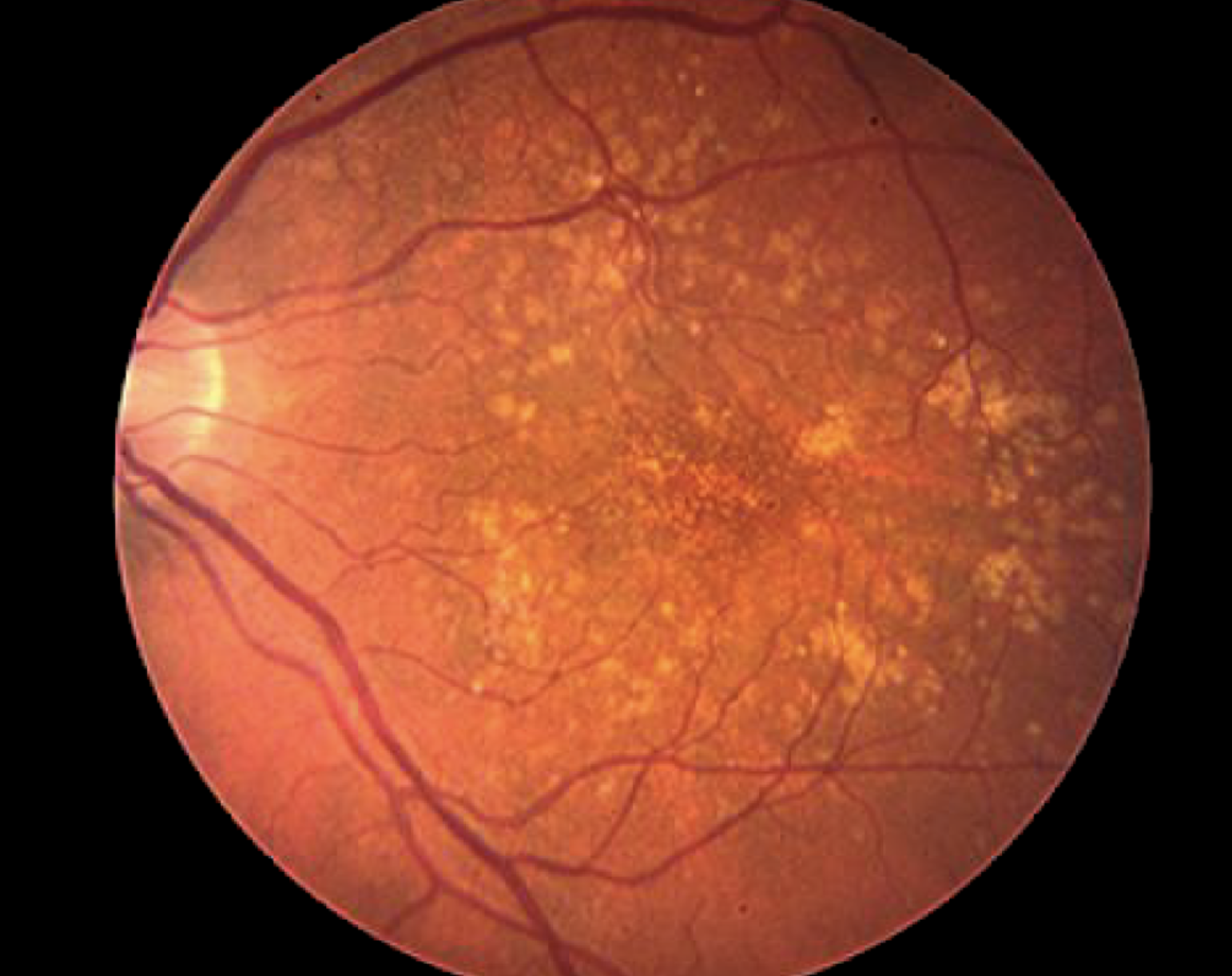
Image of an eye with geographic atrophy, an advanced and severe form of dry age-related macular degeneration.