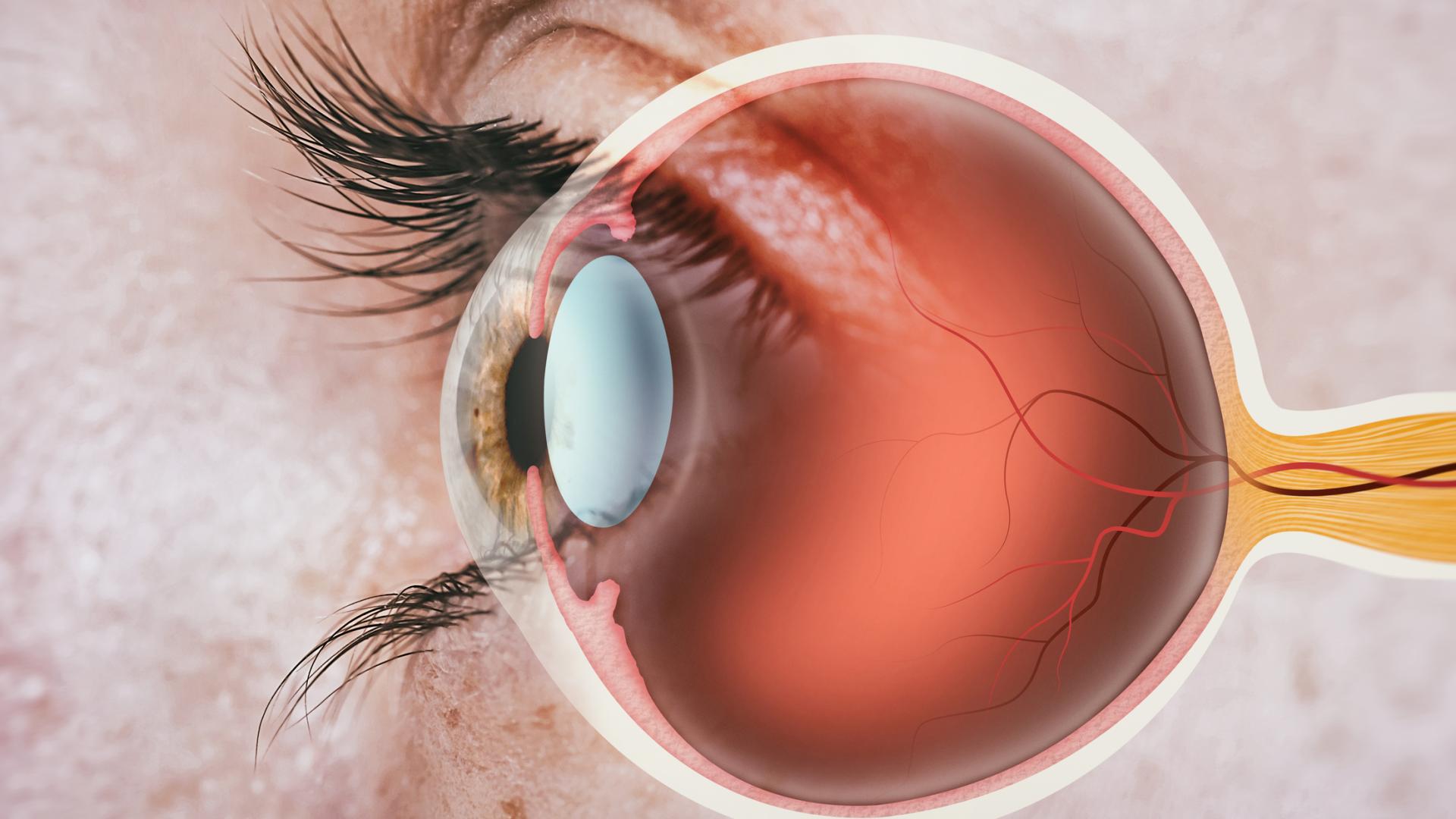
One of the new and exciting prospects in the treatment of glaucoma is called MIGS, or Minimally Invasive Glaucoma Surgeries. These procedures are exciting from a patient’s perspective because they are generally safer with quicker recovery times.
What are MIGS?
Before discussing specific MIGS procedures, it would be helpful to define what constitutes “MIGS.” There are quite a few definitions of MIGS, but the American Glaucoma Society, which is the largest society of glaucoma specialists in the United States, provides the following working definition:
- IOP, or intraocular pressure, should be lowered by improving outflow of eye fluid. This is the same mechanism used by traditional glaucoma surgeries, such as trabeculectomy or tube shunt surgeries.
- The device or procedure can either be approached from inside the eye (ab-interno) or outside the eye (ab-externo). As an example, both trabeculectomy and tube shunt procedures are performed from outside the eye, or an ab-externo approach. MIGS procedures using an approach from inside of the eye are described below in the iStent, Trabectome, Cypass (see update below), and Xen sections.
- There should be limited surgical manipulation of the sclera, which is the thick white wall of the eye. Trabeculectomy involves quite a bit of extensive cutting of the sclera, and that includes trabeculectomy modified with the Ex-PRESS shunt surgeries.
- MIGS should have little manipulation of the conjunctiva, which is the transparent outermost layer of the eye. By contrast, non-MIGS procedures, such as trabeculectomy and tube shunt procedures, involve extensive manipulation cutting of the conjunctiva.
In meeting these criteria, MIGS procedures do not preclude the possibility of future, more traditional surgical approaches, such as trabeculectomy or tube shunt surgery.
There have been a plethora of abstracts, presentations, and papers in recent years detailing the use of MIGS procedures. However, there is still a lack of absolute consensus, even among clinical trials, as to what constitutes a MIGS device, which patients are most appropriately suited for these procedures, which MIGS device is the best to use in any particular patient, and what constitutes surgical success. The purpose of this article is not to provide an exhaustive or comprehensive list and details about all of the FDA approved and not yet approved MIGS procedures that are under development, but to give patients an overview of the MIGS procedures and some questions you will want to ask your ophthalmologist to find out if a MIGS procedure is right for you.
Video: How Micro-stents Work
Safety
Overall, MIGS procedures have been touted as safer than our traditional trabeculectomy or tube shunt surgeries. This is because they do not have some of the associated complications (or have less risk) from trabeculectomy and tube shunt procedures, such as too low eye pressure (hypotony) and bleb infection. However, doctors do not yet have long-term safety or effectiveness data. Currently, there are also no completed randomized clinical trials comparing MIGS procedures to the “gold standard” procedure of trabeculectomy.
Shorter Surgery Time
Many of the MIGS procedures involve implantation of small devices. MIGS procedures are generally faster than trabeculectomy and tube shunt surgeries, and shortened surgical time can be very important for the safety of the patient. Most MIGS procedures are combined with cataract surgery, adding a short amount of time to the total surgical procedure. This is an important point to understand as well, since many clinical trials will compare a MIGS procedure combined with cataract surgery to cataract surgery alone. And indeed, cataract surgery alone has been shown to lower eye pressure to a small degree.
Lowering of Eye Pressure
Lastly, an important point is that although the majority of MIGS procedures are safer than trabeculectomy, they are also not as effective in lowering eye pressure. The eye pressure in the majority of MIGS clinical trials is typically in the mid-teens range (approximately 15 mmHg). Many ophthalmologists do not think MIGS procedures are appropriate for patients who have advanced glaucoma and need much lower eye pressures.
Trabectome
To start off a discussion of some of the currently FDA approved MIGS procedures, the Trabectome (also called trabeculectomy ab-interno) will be described, which was FDA approved in 2006. The Trabectome is an electrocautery device that removes part of the trabecular meshwork, exposing the outflow channels of the eye and thereby lowering eye pressure. The trabecular meshwork constitutes part of the drainage system of the eye, and typically the drainage of fluid is impaired in glaucoma patients, causing eye pressure to increase. The Trabectome procedure is quick and can be combined with cataract surgery. The safety profile is quite good, with the most common complication being some blood in the front of the eye after the procedure. This complication is typically limited and clears fairly quickly; however, scarring can occur over the treated area, thereby limiting the effectiveness of the Trabectome method. Furthermore, as with most MIGS procedures, the eye pressure lowering effect is limited to the mid-teens.
Another procedure that is similar to Trabectome but uses a surgical instrument (no electrocautery) to remove a strip of the trabecular meshwork. The name of the surgical instrument is called Kahook Dual Blade (KDB). An exhaustive review of all MIGS procedures is outside the scope of this article, but I bring up the example of the KDB because there are many different ways developed or still being developed to remove diseased trabecular meshwork tissue and improve outflow.
iStent
More recently, the FDA has approved the use of the iStent, which is the smallest implantable device approved for use in the human body. It is a titanium device that is implanted from inside of the eye (an ab interno approach) into the trabecular meshwork in order to bypass it and improve outflow of eye fluid. iStents are typically implanted during the time of cataract surgery, and in some cases more than one iStent is implanted. Again, the benefit of iStent is its higher safety profile, but the downside is that it does not lower eye pressure to the same extent as trabeculectomy. As with other MIGS procedures, iStent combined with cataract surgery typically lowers eye pressure to the mid-teens.
Cypass
In 2018, the manufacturer of the CyPass® micro-stent to treat glaucoma decided to voluntarily withdraw it from the market. Learn why the manufacturer took this step, and what you should do if you have already had the CyPass micro-stent procedure.
Xen
Xen is also a recently approved MIGS device that uses a gel microstent to create an ab interno trabeculectomy. In traditional trabeculectomy, a small hole is made in the eye wall, and a flap is created over it to make sure there is not too much fluid flowing out of the eye. In the case of the Xen, no incisions need to be made in the conjunctiva. Instead, the stent is placed through the eye wall and under the conjunctiva using a less invasive ab interno approach. However, the anti-scarring medications that we use with traditional trabeculectomy is often still used with Xen, thus there is no advantage of Xen over trabeculectomy in that regard. Furthermore, the Xen often requires more postoperative procedures done in the clinic because scarring still occurs.
Ongoing Clinical Trials
There are many more MIGS devices and procedures that are in clinical trials but not yet FDA approved. It does seem that the landscape for glaucoma patients is changing. The general consensus is that MIGS may be appropriate for patients who have mild to moderate glaucoma, who are intolerant of or noncompliant with eye pressure lowering drops, who do not require a very low target eye pressure, and who are undergoing cataract surgery. Because these procedures are so new, it is hard to predict exactly where MIGS will fit in to the glaucoma treatment landscape. But it is an exciting time for the field as these new technologies develop and come to market.
Questions to Ask Your Eye Doctor
- How many of these procedures have you performed?
- This question should be asked with the understanding that these procedures are quite new. While there is a learning curve in adopting any new surgical technique, MIGS procedures are designed to be quick and less complicated in terms of the number of steps required, and ophthalmologists are very adept at intraocular surgery.
- What is your personal experience with this MIGS procedures?
- Do you have a financial interest in the company that produces the MIGS device or are there any other conflicts of interest?
- Would you personally undergo this procedure or recommend a family member to have it?
About BrightFocus Foundation
BrightFocus Foundation is a premier global nonprofit funder of research to defeat Alzheimer’s, macular degeneration, and glaucoma. Since its inception more than 50 years ago, BrightFocus and its flagship research programs—Alzheimer’s Disease Research, Macular Degeneration Research, and National Glaucoma Research—has awarded more than $300 million in research grants to scientists around the world, catalyzing thousands of scientific breakthroughs, life-enhancing treatments, and diagnostic tools. We also share the latest research findings, expert information, and resources to empower the millions impacted by these devastating diseases. Learn more at brightfocus.org.
Disclaimer: The information provided here is a public service of BrightFocus Foundation and is not intended to constitute medical advice. Please consult your physician for personalized medical, dietary, and/or exercise advice. Any medications or supplements should only be taken under medical supervision. BrightFocus Foundation does not endorse any medical products or therapies.
- Glaucoma Surgery